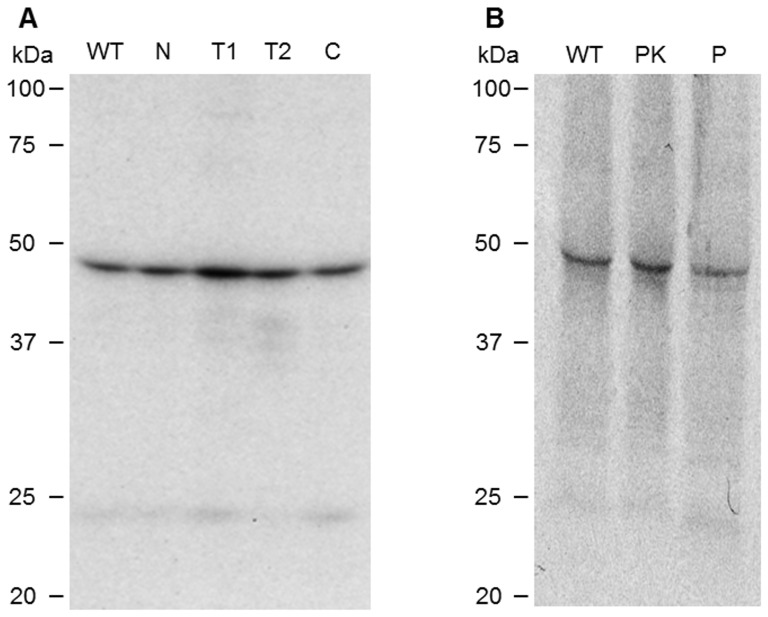
Figure 4.
A, BdRap-1 binds to a neuraminidase, trypsin and chymotrypsin-resistant RBC receptor. 35S-labelled proteins derived from parasite culture supernatants were mixed with untreated RBCs treated (WT lane), or with RBCs treated with 0.1 U/mL of Neuraminidase (N), 0.1 mg/mL trypsin (T1), 1 mg/mL trypsin (T2), and 1 mg/mL. Separated cells were then lysed and the soluble fraction was immuno-precipitated with anti-rBdRAP-1 antibodies and separated on SDS-PAGE gel. BdRAP-1 appears to be an adhesin that participates in invasion by binding to the RBC surface, as indicated by the presence of a band at the expected size of ∼46 kDa. However, none of the enzyme treatments inhibited or decreased BdRAP-1 binding, as shown the presence of the ∼46 kDa band in all lanes representing treated cells, thus, the binding profile of native BdRAP-1 to the RBC suggests the participation of a novel red cell receptor in merozoite invasion. B, BdRAP-1 binds to a non-proteinacious receptor. Binding profile of BdRAP-1 to untreated RBCs treated (WT lane), or with RBCs treated with 0.5 mg/mL of Proteinase K (PK) or 0.5 mg/mL Papain (P).