Abstract
The endogenous synthesis of arginine, a semiessential amino acid, relies on the production of citrulline by the gut and its conversion into arginine by the kidney in what has been called the “intestinal-renal axis” for arginine synthesis. Although the kidney is the main site for citrulline disposal, it only accounts for ∼60–70% of the citrulline produced. Because the only known fate for citrulline is arginine synthesis and the enzymes that catalyze this reaction are widespread among body tissues, we hypothesized that citrulline can be utilized directly by tissues to meet, at least partially, their arginine needs. To test this hypothesis, we used stable and radioactive tracers in conscious, partially nephrectomized (½ and ⅚) and anesthetized acutely kidney-ligated mouse models. Nephrectomy increased plasma citrulline concentration but did not affect citrulline synthesis rates, thus reducing its clearance. Nephrectomy (⅚) reduced the amount of citrulline accounted for as plasma arginine from 88 to 42%. Acute kidney ligation increased the half-life and mean retention time of citrulline. Whereas the rate of citrulline conversion into plasma arginine was reduced, it was not eliminated. In addition, we observed direct utilization of citrulline for arginine synthesis and further incorporation into tissue protein in kidney-ligated mice. These observations indicate that a fraction of the citrulline produced is utilized directly by multiple tissues to meet their arginine needs and that extrarenal sites contribute to plasma arginine. Furthermore, when the interorgan synthesis of arginine is impaired, these extrarenal sites are able to increase their rate of citrulline utilization.
Keywords: arginine, citrulline, kidney, stable isotope
the endogenous synthesis of arginine is an interorgan process in which its precursor, citrulline, is synthesized in the gut and, after entering the portal circulation, reaches different cell types, where it is converted into arginine. The role of the kidney as the main organ involved in the synthesis of circulating arginine has been well established (11, 14), and this process has been named the “intestinal-renal axis” for arginine synthesis (3). Using isotope tracers and arteriovenous differences, however, it has emerged that a significant fraction of the citrulline (∼30–40%) produced by the gut cannot be accounted for as plasma arginine (3, 17, 38). Because the only known fate for citrulline is its conversion into arginine and there is widespread distribution of the enzymes that catalyze the conversion of citrulline into arginine, i.e., arginine succinate synthase (ASS) and lyase (ASL) (10, 16, 37), it seems likely that plasma citrulline is utilized by different cell types directly to generate local arginine. This ability for citrulline utilization to meet the local need for arginine has been reported in endothelial cells (18), macrophages (36), and the pancreas (15) in the context of nitric oxide (NO) production and citrulline recycling. Furthermore, during sepsis there is a reduction in the “de novo” synthesis of arginine, which has been interpreted as a reduction in overall kidney function (19, 23). However, an alternative interpretation could be that some peripheral tissues increase their uptake of citrulline, thereby reducing the amount of citrulline available for plasma arginine synthesis by the kidneys. Regardless, more citrulline becomes available for direct tissue utilization during endotoxemia, and this may also contribute to tissue arginine availability and synthesis of NO.
Although in vitro studies with multiple cell types have identified the ability of citrulline to replace arginine for growth and proliferation (31, 33, 34), little information on the direct utilization of citrulline in vivo is available due to the difficulties associated with these determinations (14). The objective of this study, therefore, was to determine the magnitude of extrarenal citrulline utilization when kidney function is impaired. To accomplish this objective, three experiments were performed: in experiment 1, we determined the effect of chronically nephrectomized mice on citrulline production and disposal; in experiment 2, we established the disposal of citrulline in acutely kidney-ligated mice; and in experiment 3, we identified the conversion of citrulline into arginine and its incorporation into multiple tissues of kidney-ligated mice.
MATERIALS AND METHODS
Animals and Treatments
General.
Young adult male ICR (Institute of Cancer Research, Harlan Laboratories, Houston, TX) mice (6 wk old) were used for all the experiments. Mice were housed under standard conditions (27). All animal procedures were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee.
Experiment 1 (chronic renal nephrectomy model).
Conscious mice (initial n = 10) were studied three times: before (control, weight ± SD 30.7 ± 3.1 g) and 1 wk after ½ and ⅚ nephrectomy (1/2N and 5/6N, respectively). The weight of the mice was not affected as a result of the surgery or infusions (30.6 ± 2.9 and 31.0 ± 2.8 g, for the 1/2N and 5/6N, respectively).
SURGERY.
Mice underwent two surgical procedures, 1 wk apart. During the first procedure, the right kidney was ligated and removed to produce the 1/2N model. One week later, the left kidney was exposed and two-thirds were removed by polectomy, resulting in the 5/6N model (24).
INFUSIONS.
Mice were studied after a postsurgical 7-day recovery period. On the day of the infusion, feed was removed at 7:00, and the lateral tail vein was catheterized for infusion of tracers (25). A primed-continuous infusion of tracers was started at 10:00 and lasted for 4 h. To determine the entry rate of citrulline and arginine and the conversion of citrulline into arginine, [ureido-15N]citrulline (prime: 7 μmol/kg; continuous infusion: 7 μmol·kg−1·h−1) and [15N4]arginine (prime: 45 μmol/kg; continuous infusion: 45 μmol·kg−1·h−1) in 0.9% NaCl were infused.
SAMPLE COLLECTION.
At the end of the infusion, blood was collected from the submandibular bundle using a lancet. In addition, spot urine was collected to determine citrulline loss by this route.
Experiment 2 (acute kidney ligation).
Mice (n = 5; weight ± SD: 35.5 ± 1.9 g) were studied under anesthesia after ligation of the renal vasculature.
SURGERY.
Mice were anesthetized with 2% isoflurane in oxygen. An arterial catheter (carotid) and a venous catheter (lateral tail vein) were implanted for blood sampling and infusion, respectively. After performance of a midline laparotomy, a braided silk suture was placed around the renal vasculature of both kidneys and was either ligated (LIG) or left untied in the control group (SHAM). This procedure was completed in <25 min.
INFUSIONS.
A bolus dose of [ureido-15N]citrulline (1.2 μmol in 300 μl) was delivered with an infusion pump at a rate of 50 ml/h, followed by a continuous infusion of [15N4]arginine at a rate of 15 μmol·kg−1·h −1.
SAMPLE COLLECTION.
Approximately 15 μl of blood were collected from the arterial catheter into a preweighed 1.5-ml Eppendorf tube before and 1.5, 2.5, 4, 6, 8, 11, 15, 20, 30, 40, 50, 60, and 70 min after the start of the infusion. After weighing of the tube containing the blood sample, 80 μl of an internal standard ([U-13C6]arginine and [1,2,3,4,5 13C5]citrulline) in saline (0.9 g NaCl/l) was added, and the weight was recorded. Samples were then centrifuged, and the supernatant was kept at −80°C until analysis.
Experiment 3 (acute kidney ligation).
A preparation similar to that in experiment 2 was used (n = 5; weight ± SD: 33.6 ± 1.8 g).
INFUSIONS.
A bolus dose of [14C]citrulline (3 μCi in 300 μl saline) was delivered using the tail catheter as described above.
SAMPLE COLLECTION.
Sixty minutes after tracer administration, the mice were euthanized by decapitation and the tissues were collected to determine the incorporation of the 14C label into protein. Tissues (liver, kidney, pancreas, spleen, small and large intestine, lung, thymus, testis, heart, gastrocnemius muscle, skin, and epididymal and brown fat) were snap frozen in liquid nitrogen and weighed. Samples were stored at −80°C until analysis.
Analysis
Arginine and citrulline plasma concentrations and enrichments were determined by liquid chromatography-tandem mass spectrometry as their dansyl derivatives as described elsewhere (26). Tissue was homogenized, and protein was precipitated with perchloric acid (PCA) to determine the incorporation of 14C into protein. After the protein pellet was thoroughly washed to remove any traces of soluble intra- and extracellular contaminants, including free tracer, the pellet was solubilized with Soluene-350 (PerkinElmer, Waltham, MA) at 60°C for 4 h. Then, the scintillation cocktail Ultima Gold (PerkinElmer) was added, and 14C radioactivity was measured using a Liquid Scintillation Analyzer (Packard TriCarb 3180 TR). Tissue of animals that underwent similar surgical preparation but no [14C]citrulline infusion was used for background correction.
Calculations
For the continuous infusion of tracers (experiment 1), steady-state conditions and isotopic plateau enrichment were assumed based on our previous work (24). The rate of appearance (Ra; Eq. 1) of circulating citrulline and arginine was calculated from the isotopic dilution of the corresponding infused tracers as
| (1) |
where RaM is the Ra of the unlabeled metabolite M (μmol·kg−1·h−1), iIVM is the intravenous (iv) infusion rate of the tracer (μmol·kg−1·h−1), EiIV is the enrichment of the infused iv tracer, and EIVM is the plasma enrichment of metabolite M at isotopic plateau enrichment (mpe).
The rate of conversion (Rc; Eq. 2) of these amino acids was calculated based on the transfer of the label from the precursor to its product as previously described (25).
| (2) |
where Rcprec→prod is the rate of conversion of a precursor into its product (μmol·kg−1·h−1), Raprod is the Ra of the product, determined from the steady-state enrichments of the iv infused tracer, and Eprec and Eprod are the respective plasma enrichments of the precursor and product due to the infusion of the labeled precursor.
The conversion of infused [15N4]arginine into [15N3]citrulline is a proxy for NO synthesis, and the conversion of infused [ureido-15N]citrulline into [imino-15N]arginine was used to estimate the de novo arginine synthesis. Under steady-state conditions, the Ra of the amino acids is identical to their rate of disposal; thus clearances (ml·kg−1·min−1) were calculated by dividing the Ra by the respective plasma concentration.
For the bolus dose of [15N]citrulline (experiment 2), steady-state conditions cannot be assumed, especially in the case of the kidney-ligated animals due to the rapid expansion of the citrulline pool. For this reason, the concentration of the [15N]citrulline tracer at the different time points was calculated based on the enrichment of citrulline and plasma citrulline concentration. The following biexponential model was then applied to the resulting data
| (3) |
where yt is the concentration of tracer in blood (μmol [ureido-15N]citrulline/l) at time t, and A, b, C, and d are the parameters of the equation. The first term of the equation corresponds to distribution of citrulline within the body, and the second one to the disposal of citrulline. Goodness of fit of the model employed was determined by graphical residual analysis.
The area under the curve from zero to infinity (AUC0-∞), the area under the curve from the 0- to 70-min (AUC0–70min period of sampling and observation), and the area under the moment curve (AUMC) were calculated as in the following
| (4) |
| (4') |
and
| (5) |
where AUC is expressed as [ureido-15N]citrulline·l−1·min−1); AUMC is expressed [ureido-15N]citrulline·l−1·min−2); A, b, C, and d are the parameters calculated previously; and E is the [ureido-15N]citrulline concentration at 70 min.
The terminal half-life (λ) after reaching pseudoequilibrium (min) and mean residence time (MRT; min) were calculated as
| (6) |
and
| (7) |
Because of the different kinetics between the LIG and SHAM groups for the arginine produced from the citrulline bolus dose, no single equation could be fitted. Thus the trapezoidal rule was utilized to obtain the AUC between 0 and 70 min for the 15N and 15N4 arginine data. The actual amount of arginine produced from the citrulline bolus dose can be approximated as follows
| (8) |
where AUC15NArg and AUC15N4Arg are the AUC for the arginine produced from citrulline and the arginine infused, respectively, and infused15N4Arginine is the total amount of arginine infused during the experiment. Note that this equation does not represent a precursor-product relationship as Eq. 2. It represents the ratio between the AUC for two isotopologs of arginine, and because one was infused (and thus the total amount infused is known), the total conversion of the bolus [15N]citrulline to [15N]arginine can then be estimated.
Statistical Analysis
Results from the chronic renal nephrectomy model were analyzed as a mixed model with mouse as the random variable of the model using the “proc MIXED” procedure of SAS (v. 9.2, SAS, Cary, NC). In this way, comparisons were made within animals. For the acute kidney ligation models, the “proc NLIN” procedure was utilized to calculate the parameters of the biexponential equation. Homogeneity of the variances was tested using Levene's test; for those variables with heterogeneous variance, a log transformation of the variable was performed before statistical analysis. For these variables, the mean and confidence intervals are presented. Otherwise, means ± SE are reported.
RESULTS
Arginine and Citrulline Kinetics in Chronically Nephrectomized Mice
Partial nephrectomy reduced the clearance rate of arginine and citrulline and increased the plasma concentration of these amino acids without affecting their rates of appearance (Table 1). The rate of conversion of arginine to citrulline, a proxy for NO production, was not different among the three treatments (Table 1). The conversion of citrulline to arginine (de novo arginine production), which was 88% of the citrulline produced in control mice, was reduced to 64 and 42% in nephrectomized animals (1/2N and 5/6N, respectively). Only traces of citrulline were found in urine regardless of the extent of kidney ablation.
Table 1.
Citrulline and arginine kinetics in control and nephrectomized mice
| Control | 1/2N | 5/6N | P | |
|---|---|---|---|---|
| Plasma concentration, μmol/l | ||||
| Arginine | 37.7 ± 4.0b | 32.5 ± 4.8b | 119.4 ± 32.6a | <0.0002 |
| Citrulline | 20.4 ± 1.8b | 37.4 ± 3.6b | 111.0 ± 34.9a | <0.0003 |
| Rate of appearance, μmol·kg−1·h−1 | ||||
| Arginine | 434.1 ± 25.6 | 406.8 ± 36.0 | 504.2 ± 37.0 | <0.2112 |
| Citrulline | 121.5 ± 5.0 | 116.5 ± 11.5 | 126.5 ± 11.6 | <0.7976 |
| Rate of conversion, μmol·kg−1·h−1 | ||||
| Arginine to citrulline | 0.88 ± 0.12 | 0.74 ± 0.06 | 1.09 ± 0.06 | <0.0943 |
| Citrulline to arginine | 107.9 ± 11.9a | 74.9 ± 14.6ab | 53.8 ± 9.3b | <0.0430 |
| Clearance, ml·kg−1·min−1 | ||||
| Arginine | 197.7a | 228.7a | 83.2b | <0.0105 |
| Citrulline | 103.1 ± 6.5a | 55.7 ± 6.4b | 30 ± 10.6b | <0.0001 |
Values are means ± SE; control and ½ nephrectomy (½N), n = 10; ⅚ nephrectomy (⅚N), n = 7. For arginine clearance, the variance was not homogenous across treatments, and thus this variable was log transformed. 95% confidence intervals were 166–236, 146–357, and 45-154 ml·kg−1·h−1 for control, 1/2N, and 5/6N treatment, respectively.
Values with different superscripts differ (P < 0.05).
Citrulline Disposal During Acute Kidney Ligation
Kidney ligation increased (P < 0.001) plasma citrulline concentration (Fig. 1) and reduced the rate of disposal of the citrulline tracer injected (Fig. 2). This resulted in a greater AUC of the [15N]citrulline tracer and a longer half-life and MRT of the citrulline pool in the LIG mice (Table 2). The ligation of the kidneys resulted in a 65 and 84% increase in these two parameters, respectively. The appearance of [imino-15N]arginine (resulting from [ureido-15N]citrulline) followed a different pattern in the two treatment groups (Fig. 3). In the SHAM group [15N]arginine appeared almost immediately after the citrulline tracer was administered, and its plasma concentration declined over time. In the LIG group, [15N]arginine appeared in a slow, but steady fashion. Whereas the AUC for the [15N4]arginine tracer continuously infused was not different between the two groups (P = 0.597, data not shown), the AUC for [15N]arginine in the LIG group was ∼24% of the area calculated for the SHAM group (Table 2). This translated into a reduction in the 15N label recovered in arginine in the LIG mice. The percentage of the infused 15N label recovered as [15N]arginine was 14.2 and 61.7% for the LIG and SHAM group, respectively. This represented a fivefold increase (from 13.6 to 72.7 μmol·kg−1·h−1) in the amount of citrulline disposed by extra renal tissues.
Fig. 1.
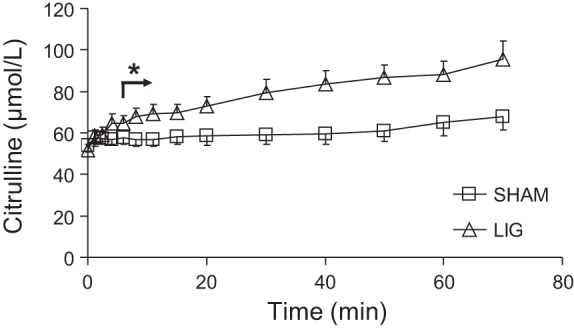
Plasma citrulline concentration in mice after kidney ligation (LIG) or sham surgery (SHAM). Values are means ± SE; n = 5. Arrow and *P < 0.05, for treatment differences.
Fig. 2.
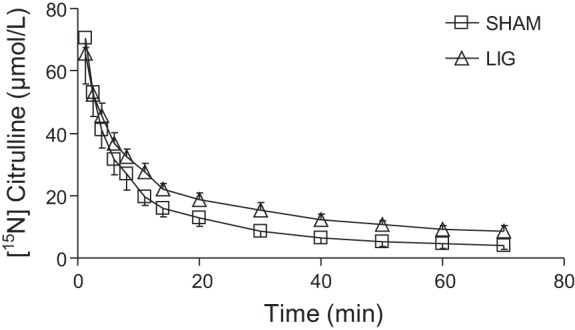
Plasma [ureido-15N]citrulline concentration after a bolus dose of this tracer in mice that underwent kidney ligation (LIG) or SHAM surgery. Biexponential curves were fitted to the data (LIGt = 49.6 ± 2.4·e(−0.27 ± 0.03·t) + 26.7 ± 2.2·e(−0.02 ± 0.00·t), R2 = 0.98; LIGt = 59.8 ± 1.1·e(−0.22 ± 0.01·t) + 16.3 ± 1.3·e(−0.03 ± 0.00·t), R2 = 0.94).
Table 2.
[ureido-15N]citrulline and [15N4]arginine kinetics in sham (SHAM) and kidney-ligated (LIG) mice
| SHAM | LIG | P | |
|---|---|---|---|
| [ureido-15N]citrulline | |||
| AUC0-∞, μmol/min | 1,012 ± 98 | 1,726 ± 155 | <0.011 |
| AUC0–70 min, μmol/min | 998 ± 99 | 1,588 ± 77 | <0.003 |
| AUMC, μmol/min2 | 27,340 ± 5,076 | 86,845 ± 15,310 | <0.021 |
| λ, min | 23.5 ± 3.0 | 38.7 ± 3.3 | <0.014 |
| MRT, min | 26.7 ± 3.5 | 49.2 ± 4.3 | <0.007 |
| [15N]arginine | |||
| AUC0–70 min, μmol/min | 351 ± 8.5 | 85 ± 3.6 | <0.001 |
| Recovered, μmol | 0.74 ± 0.06 | 0.17 ± 0.01 | <0.001 |
| As % infused [15N]citrulline | 61.7 ± 5.22 | 14.2 ± 0.48 | <0.001 |
| [15N4]arginine | |||
| AUC0–70 min, μmol/min | 297 ± 18 | 315 ± 27.2 | <0.597 |
Values are means ± SE; n = 5.
AUC, area under the curve; AUMC, area under the moment curve; λ, half-life; MRT, mean retention time.
Fig. 3.
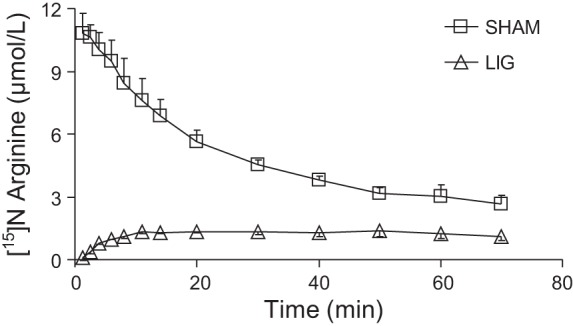
Plasma [15N]arginine concentration after a bolus dose of [ureido-15N]citrulline in LIG or SHAM mice.
Citrulline Utilization in Kidney-Ligated Mice
The 14C label originally in citrulline was incorporated to a different extent in the different tissues analyzed in the LIG and SHAM group (Fig. 4). In the SHAM group, the pancreas and kidney had the highest level of 14C incorporation. Kidney ligation reduced (P < 0.05) the incorporation of radioactivity in some tissues (spleen, stomach, small and large intestine, thymus, heart, gastrocnemius muscle, brain, and brown adipose tissue), with no incorporation in the kidney as expected. There was no difference between the SHAM and LIG groups for liver (P = 0.211), pancreas (P = 0. 485), lungs (P = 0.201), testis (P = 0.933), skin (P = 0.372), and epididymal fat pad (P = 0.478).
Fig. 4.
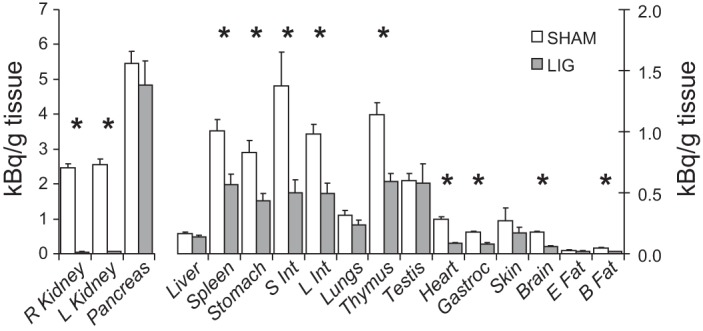
Tissue protein 14C radioactivity after a bolus dose of [ureido-14C]citrulline in LIG or SHAM mice. Values are means ± SE; n = 5. *P < 0.05, for treatment differences.
DISCUSSION
The synthesis of citrulline from ornithine takes place in only two cell types, the enterocyte and the hepatocyte. In the liver, citrulline functions as part of the urea cycle in the detoxification of ammonia, and because of the channeling of urea cycle intermediates (7), little or no citrulline escapes the liver. The citrulline produced in the small intestine, however, enters the portal vein, escapes liver extraction, and appears in the peripheral circulation, serving as a precursor for arginine synthesis. The contribution of other pathways such as NO synthesis, degradation of citrullinated proteins, and demethylation of methylarginines are likely to contribute a small fraction of the total flux of citrulline. Because most of the circulating citrulline is of enteral origin, plasma citrulline concentration has been proposed as a marker for gut mass and function (9, 32). Conversely, because citrulline utilization takes place mainly in the kidney, plasma citrulline can also be considered a marker of renal function (21). Whereas the determination of citrulline synthesis rate and its precursors is relatively straightforward (25), the determination of citrulline disposal and utilization is more complex. For this reason, we utilized two different and complementary approaches. In chronically nephrectomized mice, the rate of appearance of citrulline was determined, which under the (pseudo) steady-state conditions of the experiment is equivalent to its rate of disposal. In kidney-ligated mice, a bolus dose of labeled citrulline was administered and the rate of disposal of the tracer (and tracee) was determined directly.
The role of the kidney has been established by arteriovenous differences in rats (11) and dogs (38). However, a significant fraction of citrulline disposal (∼40%) cannot be accounted for by renal metabolism (38). Similarly, the de novo arginine production determined with stable isotopes fails to account for 25–40% of the rate of appearance of citrulline (4, 29). Because the only known metabolic fate of citrulline is its conversion into arginine (through argininosuccinate), it is likely that this unaccounted fraction is utilized for arginine synthesis in “hidden” compartments that do not equilibrate with plasma. Initially, to study the nonrenal disposal of citrulline, we crossed ASLflox/flox mice (13) with an improved Cre recombinase (iCre) under the control of the kidney androgen-regulated protein (KAP) promoter (22). However, we were unable to alter the de novo synthesis of arginine with this model, even after providing exogenous testosterone to male mice. Subsequent immunohistochemical analysis showed that ASL was still present in the proximal tubules of the kidney. For this reason, we resorted to other well-established models of kidney function impairment to disrupt the interorgan metabolism of citrulline and arginine. These models, however, have some limitations: chronic partial nephrectomy is a well-established model for kidney disease, and this may have an independent impact on arginine metabolism. Acute kidney ligation is usually done under general anesthesia, which also can have an effect on the metabolism of citrulline and arginine. Regardless, the main limitation of this approach is that the disappearance of citrulline is what is being measured and not its utilization for arginine synthesis. For this reason, we used a complementary approach to determine the tissue utilization of citrulline for arginine synthesis when renal function is impaired. To determine this local process, we measured the incorporation of the ureido carbon of citrulline into the acid-precipitable fraction of different tissues. This approach assumes that 1) there is no tRNA for citrulline and thus no (direct) incorporation of citrulline into protein; 2) the new arginine that is formed from citrulline can then be incorporated into protein, and, in kidney-ligated mice, this process reflects a local phenomenon; 3) protein is the main component of the acid-precipitable fraction; and 4) the main fate of the ureido carbon of citrulline is urea, which is lost from the system, i.e., the ureido carbon does not recycle (this is also true for other minor pathways, e.g., agmatine and creatine synthesis).
Chronic Nephrectomy Reduces Clearance of Citrulline and De Novo Synthesis of Arginine
Given the central role of the kidney in the disposal of citrulline (11, 14), the increase in plasma citrulline concentration following partial nephrectomy was expected (1, 5). Although the increase in plasma arginine concentration seems counterintuitive, this phenomenon has been previously reported in mice (1).The observed increase in plasma citrulline concentration in chronically nephrectomized animals was due to impaired citrulline disposal, because the rate of appearance of citrulline was unaffected (Table 1). However, the rate of disposal of citrulline has to match the rate of appearance to maintain a (pseudo) steady state. The disposal of citrulline takes place by conversion into argininosuccinate and then arginine or alternatively by excretion into the urine. We have only detected traces of citrulline in the urine, which is consistent with previous observations in partially nephrectomized mice (1) and with the great ability of the kidney to reabsorb citrulline after an overload with this amino acid (30). Thus, in the absence of urinary citrulline excretion, it seems likely that citrulline was utilized for arginine synthesis by nonrenal tissue. Chronic partial nephrectomy reduced the efficiency with which citrulline was removed from the system, as indicated by the increase in plasma citrulline concentration and the decrease in its clearance rate. The amount of citrulline recovered as plasma arginine (de novo arginine synthesis) was also reduced in this nephrectomy model, and the amount disposed of by extrarenal tissues increased fivefold. The conversion of arginine into citrulline, a proxy for NO production, was not affected by partial nephrectomy in this study. Other authors have reported a decrease in the production of NO in chronic kidney disease (2, 6). The reason for the discrepancy between our data and previous reports may be due to the higher plasma arginine concentration observed in our animals, or that more time after the renal injury is needed for the development of a full renal dysfunction model to become established.
Acute Kidney Ligation Increases the Half-Life of Citrulline
The abrupt ligation of the kidneys also resulted in a reduction in the disposal of citrulline, which translated into a greater plasma concentration, AUC, longer half-life, and residence time of this amino acid (Fig. 1 and Table 2). The appearance of [15N]arginine in blood in the LIG mice (Fig. 3) indicates that nonrenal tissues produce and export arginine synthesized from plasma citrulline. Assuming that this process is also present in the SHAM group, we can estimate that this nonrenal source accounts for ∼24% of de novo arginine synthesis. The amount of citrulline infused, recovered as plasma arginine in the SHAM group, was ∼60%, which falls within the range previously reported (28). For the LIG group, however, only 14% of the citrulline infused was recovered as plasma arginine (Table 2). This nonrenal source of plasma arginine, to the best of our knowledge, has not been described previously.
Plasma Citrulline Is Incorporated in Tissues Despite the Presence of a Metabolically Active Kidney
The ureido-14C originally in the citrulline administered was readily incorporated into the acid-precipitable fraction of multiple tissues in both the LIG and SHAM groups. In animals without a functional kidney, this utilization represents the uptake of plasma citrulline, its conversion into arginine, and, finally, its incorporation into protein. The difference in incorporation among the different tissues is likely to represent (for the most part) their rate of protein synthesis, rather than their ability to convert citrulline into arginine. Nonetheless, these measurements indicate that the conversion of citrulline into arginine is widely present among body tissues. Interestingly, a reduced incorporation was found in the liver of SHAM and LIG mice, despite its high protein synthesis rate; this response is consistent with the poor uptake of citrulline by this organ reported by early researchers (8, 20). Not surprisingly, LIG mice had a reduced 14C incorporation into protein in some tissues, since these animals had to rely on the local conversion of citrulline to arginine, whereas SHAM mice in addition were also able to utilize plasma [14C]arginine. The pancreas, however, despite a very high fractional protein synthesis rate (>400%/day, Marini JC, unpublished observations), seems to be able to produce enough arginine from citrulline to sustain its high protein synthesis rate.
In conclusion, when taken together, the data indicate that a fraction of the citrulline produced is utilized directly by multiple tissues to meet their arginine needs and that extrarenal sources contribute to plasma arginine. Furthermore, when the interorgan synthesis of arginine is impaired due to nephrectomy or kidney ligation, these extrarenal sites are able to increase their rate of citrulline utilization. This direct citrulline utilization may be the reason why citrulline supplementation has been more effective than arginine supplementation in improving the microcirculation during endotoxemia (35) and restoring NO synthesis in patients with mitochondrial disorders (12).
GRANTS
This work was supported by funds from the US Department of Agriculture, Agricultural Research Service, under Cooperative Agreement Number 58-6250-6-001, and the National Institutes of Health (Grant K01 RR024173 to J. C. Marini).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.C.M. provided conception and design of research; J.C.M., I.C.D., and M.L.F. performed experiments; J.C.M. analyzed data; J.C.M. and M.L.F. interpreted results of experiments; J.C.M. prepared figures; J.C.M. drafted manuscript; J.C.M. and M.L.F. edited and revised manuscript; J.C.M., I.C.D., and M.L.F. approved final version of manuscript.
REFERENCES
- 1.Al Banchaabouchi M, Marescau B, D'Hooge R, Engelborghs S, De Deyn PP. Consequences of renal mass reduction on amino acid and biogenic amine levels in nephrectomized mice. Amino Acids 18: 265–277, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Baylis C. Nitric oxide deficiency in chronic kidney disease. Am J Physiol Renal Physiol 294: F1–F9, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Brosnan ME, Brosnan JT. Renal arginine metabolism. J Nutr 134: 2791S-2795S, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Castillo L, Chapman TE, Sanchez M, Yu YM, Burke JF, Ajami AM, Vogt J, Young VR. Plasma arginine and citrulline kinetics in adults given adequate and arginine-free diets. Proc Natl Acad Sci USA 90: 7749–7753, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen GF, Baylis C. In vivo renal arginine release is impaired throughout development of chronic kidney disease. Am J Physiol Renal Physiol 298: F95–F102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen GF, Moningka NC, Sasser JM, Zharikov S, Cunningham M, Jr, Tain YL, Schwartz IF, Baylis C. Arginine and asymmetric dimethylarginine in puromycin aminonucleoside- induced chronic kidney disease in the rat. Am J Nephrol 35: 40–48, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung CW, Cohen NS, Raijman L. Channeling of urea cycle intermediates in situ in permeabilized hepatocytes. J Biol Chem 264: 4038–4044, 1989 [PubMed] [Google Scholar]
- 8.Cohen PP, Hayano M. The conversion of citrulline to arginine (transimination) by tissue slices and homogenates. J Biol Chem 239–250, 1946 [PubMed] [Google Scholar]
- 9.Crenn P, Coudray-Lucas C, Thuillier F, Cynober L, Messing B. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology 119: 1496–1505, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Daniel EE, Wang YF, Salapatek AM, Mao YK, Mori M. Arginosuccinate synthetase, arginosuccinate lyase and NOS in canine gastrointestinal tract: Immunocytochemical studies. Neurogastroenterol Motil 12: 317–334, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Dhanakoti SN, Brosnan JT, Herzberg GR, Brosnan ME. Renal arginine synthesis: studies in vitro and in vivo. Am J Physiol Endocrinol Metab 259: E437–E442, 1990 [DOI] [PubMed] [Google Scholar]
- 12.El-Hattab AW, Hsu JW, Emrick LT, Wong LJC, Craigen WJ, Jahoor F, Scaglia F. Restoration of impaired nitric oxide production in MELAS syndrome with citrulline and arginine supplementation. Mol Genet Metab 105: 607–614, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erez A, Nagamani SCS, Shchelochkov OA, Premkumar MH, Campeau PM, Chen Y, Garg HK, Li L, Mian A, Bertin TK, Black JO, Zeng H, Tang Y, Reddy AK, Summar M, O'Brien WE, Harrison DG, Mitch WE, Marini JC, Aschner JL, Bryan NS, Lee B. Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat Med 17: 1619–1626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Featherston WR, Rogers QR, Freedland RA. Relative importance of kidney and liver in synthesis of arginine by the rat. Am J Physiol 224: 127–129, 1973 [DOI] [PubMed] [Google Scholar]
- 15.Flodstrom M, Niemann A, Bedoya FJ, Morris SM, Eizirik DL. Expression of the citrulline-nitric oxide cycle in rodent and human pancreatic beta-cells - induction of argininosuccinate synthetase by cytokines. Endocrinology 136: 3200–3206, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Haines RJ, Pendleton LC, Eichler DC. Argininosuccinate synthase: At the center of arginine metabolism. Int J Biochem Mol Biol 2: 8–23, 2011 [PMC free article] [PubMed] [Google Scholar]
- 17.Hallemeesch MM, Cobben DCP, Dejong CHC, Soeters PB, Deutz NEP. Renal amino acid metabolism during endotoxemia in the rat. J Surg Res 92: 193–200, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Hecker M, Sessa WC, Harris HJ, Anggard EE, Vane JR. The metabolism of l-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle l-citrulline to l-arginine. Proc Natl Acad Sci USA 87: 8612–8616, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kao CC, Bandi V, Guntupalli KK, Wu M, Castillo L, Jahoor F. Arginine, citrulline and nitric oxide metabolism in sepsis. Clin Sci 117: 23–30, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Krebs HA, Henseleit K. Untersuchungen uber die Harnstoffbildung im Tierkorper. Z Physiol Chem 33–66, 1932 [Google Scholar]
- 21.Levillain O, Parvy P, Hassler C. Amino acid handling in uremic rats: citrulline, a reliable marker of renal insufficiency and proximal tubular dysfunction. Metabolism 46: 611–618, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Li H, Zhou X, Davis DR, Xu D, Sigmund CD. An androgen-inducible proximal tubule-specific Cre recombinase transgenic model. Am J Physiol Renal Physiol 294: F1481–F1486, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luiking YC, Poeze M, Ramsay G, Deutz NEP. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am J Clin Nutr 89: 142–152, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Ma LJ, Fogo AB. Model of robust induction of glomerulosclerosis in mice: importance of genetic background. Kidney Int 64: 350–355, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Marini JC. Arginine and ornithine are the main precursors for citrulline synthesis in mice. J Nutr 142: 572–580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marini JC. Quantitative analysis of 15N-labeled positional isomers of glutamine and citrulline via electrospray ionization tandem mass spectrometry of their dansyl derivatives. Rapid Commun Mass Spectrom 25: 1291–1296, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Marini JC, Didelija IC, Castillo L, Lee B. Glutamine: precursor or nitrogen donor for citrulline synthesis? Am J Physiol Endocrinol Metab 299: E69–E79, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marini JC, Didelija IC, Castillo L, Lee B. Plasma arginine and ornithine are the main citrulline precursors in mice infused with arginine-free diets. J Nutr 140: 1432–1437, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marini JC, Stoll B, Didelija IC, Burrin DG. De novo synthesis is the main source of ornithine for citrulline production in neonatal pigs. Am J Physiol Endocrinol Metab 303: E1348–E1353, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moinard C, Nicolis I, Neveux N, Darquy S, Benazeth S, Cynober L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: the Citrudose pharmacokinetic study. Br J Nutr 99: 855–862, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Morgan JF, Morton HJ, Pasieka AE. The arginine requirement of tissue cultures. I. Interrelationships between arginine and related compounds. J Biol Chem 233: 664–667, 1958 [PubMed] [Google Scholar]
- 32.Rhoads JM, Plunkett E, Galanko J, Lichtman S, Taylor L, Maynor A, Weiner T, Freeman K, Guarisco JL, Wu GY. Serum citrulline levels correlate with enteral tolerance and bowel length in infants with short bowel syndrome. J Pediatr 146: 542–547, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Sun NC, Sun CRY, Tennant RW, Hsie AW. Selective growth of some rodent epithelial-cells in a medium containing citrulline. Proc Natl Acad Sci USA 76: 1819–1823, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tytell AA, Neuman RE. Growth response of stable and primary cell cultures to l-ornithine, l-citrulline, and l-arginine. Exp Cell Res 20: 84–91, 1960 [DOI] [PubMed] [Google Scholar]
- 35.Wijnands KAP, Vink H, Briedé JJ, van Faassen EE, Lamers WH, Buurman WA, Poeze M. Citrulline a more suitable substrate than arginine to restore NO production and the microcirculation during endotoxemia. PLoS ONE 7: e37479, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu GY, Brosnan JT. Macrophages can convert citrulline into arginine. Biochem J 281: 45–48, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu Y, Terada K, Nagasaki A, Takiguchi M, Mori M. Preparation of recombinant argininosuccinate synthetase and argininosuccinate lyase: Expression of the enzymes in rat tissues. J Biochem (Tokyo) 117: 952–957, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Yu YM, Burke JF, Tompkins RG, Martin R, Young VR. Quantitative aspects of interorgan relationships among arginine and citrulline metabolism. Am J Physiol Endocrinol Metab 271: E1098–E1109, 1996 [DOI] [PubMed] [Google Scholar]


