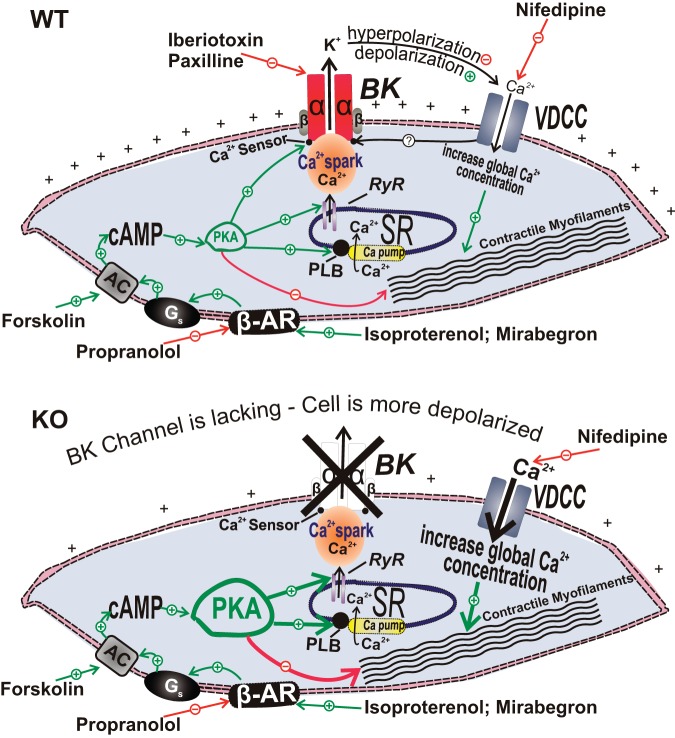
Fig. 3.
Illustration of the cellular mechanisms by which BK channels mediate β-adrenergic relaxation in UBSM cells with demonstration of the differential outcomes in the wild-type (WT) mouse (top) and the BK-knockout (KO) mouse (bottom), respectively. In UBSM cells from WT mice, functionally active BK channels regulate Ca2+ entry via L-type voltage-dependent Ca2+ channels (VDCC), and, thus, contractility (top). In addition, BK channels are under the local control of the so-called “Ca2+ sparks” caused by Ca2+ release from the ryanodine receptors of the sarcoplasmic reticulum, adjacent to the cell membrane. Following permanent BK channel pore forming α-subunit gene deletion in the BK channel KO mouse, an adaptive compensatory upregulation of the β-AR/cAMP/PKA signaling pathway develops. The enhanced β-AR/PKA activity compensates for the increased Ca2+ entry via L-type VDCC that occurs due to sustained membrane depolarization in the absence of the BK channels. AC, adenylyl cyclase; BK, large-conductance voltage- and Ca2+-activated K+ channels; β-AR, β-adrenergic receptors; VDCC, L-type voltage-dependent Ca2+ channels; Gs, stimulatory G protein; PKA, protein kinase-A; PLB, phospholamban; PLC, phospholipase C; RyR, ryanodine receptor; SR, sarcoplasmic reticulum. This figure is based on information from Brown et al. (21).