Abstract
α-Tocopherol blocks responses to allergen challenge in allergic adult mice, but it is not known whether α-tocopherol regulates the development of allergic disease. Development of allergic disease often occurs early in life. In clinical studies and animal models, offspring of allergic mothers have increased responsiveness to allergen challenge. Therefore, we determined whether α-tocopherol blocked development of allergic responses in offspring of allergic female mice. Allergic female mice were supplemented with α-tocopherol starting at mating. The pups from allergic mothers developed allergic lung responses, whereas pups from saline-treated mothers did not respond to the allergen challenge, and α-tocopherol supplementation of allergic female mice resulted in a dose-dependent reduction in eosinophils in the pup bronchoalveolar lavage and lungs after allergen challenge. There was also a reduction in pup lung CD11b+ dendritic cell subsets that are critical to development of allergic responses, but there was no change in several CD11b− dendritic cell subsets. Furthermore, maternal supplementation with α-tocopherol reduced the number of fetal liver CD11b+ dendritic cells in utero. In the pups, there was reduced allergen-induced lung mRNA expression of IL-4, IL-33, TSLP, CCL11, and CCL24. Cross-fostering pups at the time of birth demonstrated that α-tocopherol had a regulatory function in utero. In conclusion, maternal supplementation with α-tocopherol reduced fetal development of subsets of dendritic cells that are critical for allergic responses and reduced development of allergic responses in pups from allergic mothers. These results have implications for supplementation of allergic mothers with α-tocopherol.
Keywords: allergic lung inflammation, vitamin E
development of allergic disease originates as complex environmental and genetic interactions that may start early in life (49), a time when the airways and the immune system are developing. In reports examining human maternal and paternal asthma associations with development of allergies in offspring, most associations are with maternal asthma (46). In animal studies, offspring from allergic mothers are predisposed to responses to suboptimal doses of allergens, and this risk is sustained to 8 wk of age in the mouse (26, 28–30, 32, 33, 36, 46). Interestingly, in animal studies, dendritic cells (DCs) from offspring of allergic mothers transfer the risk for allergies to naïve neonates, indicating a functional change in neonatal DCs during neonate development in allergic mothers (24). It is suggested that risk for allergic disease in humans is associated with in utero and early exposures to environmental factors (9).
The prevalence of asthma from 1950 to the present has increased in many countries (10, 27, 76). In humans, the marked differences in rates of asthma within countries, in migrating populations, and over relatively short periods of time support an important role of exposure to the local environment, such as diet, in asthma inception. It is reported that patients with asthma or allergy have low levels of the vitamin E isoform α-tocopherol (38, 39, 63, 64). Therefore, an increase in α-tocopherol may be necessary, in combination with other regimens, to decrease allergic disease. Studies with vitamin E report seemingly conflicting outcomes for allergy and other inflammatory diseases (19, 22, 65, 70, 79). We recently demonstrated that different isoforms of vitamin E have opposing functional outcomes in lungs in animals and humans (1, 7, 16, 17, 19, 48, 50).
Vitamin E consists of natural isoforms and synthetic racemic isoforms. The four natural tocopherol isomers are d-α-, d-β-, d-γ-, and d-δ-tocopherol. The most abundant isoforms are α-tocopherol and γ-tocopherol, which differ by one methyl group. Plants synthesize the natural isoforms from tyrosine and chlorophyll (34). Mammals consume the plant tocopherols in food and cooking oils, and mammals do not interconvert the isoforms. Dietary tocopherols are taken up from the intestine, transported via the lymph to the blood and then to the liver. In the liver, α-tocopherol transfer protein (αTTP) preferentially transfers α-tocopherol to lipoproteins, resulting in 10-fold higher α-tocopherol than γ-tocopherol in tissues (81). Tocopherols in plasma lipoproteins are transferred to cells by plasma phospholipid transfer protein, scavenger receptors, or the lipoprotein lipase pathway (21). After uptake, the tocopherols are found in cell membranes, since they are lipid vitamins. αTTP is also expressed by trophoblast, fetal endothelium, and amnion epithelium of the placenta (54). It is reported that basal levels of α-tocopherol are required for placentation (37, 54).
We have demonstrated in adults that α-tocopherol is anti-inflammatory and γ-tocopherol is proinflammatory (7, 20, 50). When presensitized allergic adult mice are supplemented with α-tocopherol and then challenged with antigen, allergic inflammation is decreased 65%, whereas γ-tocopherol supplementation increases allergic inflammation by 175% (7). In light of opposing functions for tocopherol isoforms, we have reviewed alternative interpretations of the seemingly conflicting reports on vitamin E and the vitamin E isoforms in these studies (1, 15, 19, 20). Furthermore, we have demonstrated that, in humans, α-tocopherol associates with better lung spirometry, and γ-tocopherol associates with lower lung spirometry by age 21 (48), suggesting that tocopherol isoforms may regulate development and lung responses to environmental pollutants, allergens, or infections. It is not known whether development of allergic disease is regulated by vitamin E. Therefore, we hypothesized that maternal supplementation with α-tocopherol inhibits allergic responses in the offspring.
We report that supplementation of allergic female mice with α-tocopherol inhibited neonatal development of allergic responses. In addition, there was a reduction in neonatal cytokines, chemokines, and lung CD11b+ dendritic cell subsets, which are critical to development of allergic responses. α-Tocopherol also reduced CD11b+ dendritic cell subsets in the fetal liver and in vitro in bone marrow differentiated DCs. These data have important implications for supplementation of allergic mothers with α-tocopherol to limit development of allergic disease in offspring.
MATERIALS AND METHODS
Animals.
C57BL/6 mice were from Jackson Laboratories (Bar Harbor, ME). The studies are approved by the Northwestern University Institutional Review Committee for animals.
Tocopherol reagents.
d-α-Tocopherol (>98% pure) from Sigma was sent to Dyets (Bethlehem, PA) to make the diets with 150 mg α-tocopherol/kg diet (catalog no. 103372), 250 mg α-tocopherol/kg diet (catalog no. 103373), and 500 mg α-tocopherol/kg diet (catalog no. 103296) (Table 1). The purity of these tocopherols that were used to make the diets and the tocopherol concentrations in the diets was confirmed by HPLC with electrochemical detection as described below.
Table 1.
α-Tocopherol-supplemented diets (modified AIN-93G Purified Rodent Diet with corn oil replacing soybean oil)
| g/kg diet | |
|---|---|
| Casein, high nitrogen | 200 |
| l-Cystine | 3 |
| Sucrose | 100 |
| Cornstarch | 397 |
| Dyetrose | 132 |
| Corn oil | 70 |
| Cellulose | 50 |
| Mineral mix no. 210025 | 35 |
| Vitamin mix no. 210025 | 10 |
| Choline bitartrate | 2.5 |
| d-α-tocopherol | 0.5, 0.25, or 0.15 |
The α-tocopherol-supplemented diet (500 mg/kg diet no. 103296, 250 mg/kg diet no. 103373, or 150 mg/kg diet no. 103372) and the basal control diet without supplementation (45 mg α-tocopherol/kg diet) (diet no. 101591) were from Dyets. Corn oil, which was commonly used in rodent diet in the past, was used in this diet instead of the more recent formulas with soybean oil to avoid the proinflammatory contribution of high γ-tocopherol in soybean oil as we have reported (5, 17, 44).
Ovalbumin/tocopherol administration and inflammation.
C57BL/6 female mice were maintained on chow diet. The mice were sensitized by intraperitoneal injection (200 μl) of ovalbumin (OVA) grade V (5 μg)/potassium aluminum sulfate (alum, 1 mg) or saline/alum (1 mg) on days 0 and 7 (26, 28, 46). The mice were exposed to nebulized saline or 3% (wt/vol) OVA in saline for 15 min on three consecutive days at 8, 12, and 16 wk of age. At 18 wk old, the female mice were challenged one time with 3% OVA (26, 28, 46) and then fed basal diet or diet supplemented with d-α-tocopherol (500 mg α-tocopherol/kg diet) (Table 1) (42, 52, 71). Basal rather than deficient tocopherol diets are used because α-tocopherol is necessary for placental development (37, 54). The 250 to 500 mg d-α-tocopherol/kg diet doses were chosen for these studies because it is commonly used to elevate tissue tocopherols and regulate immune responses in adult rodents (52, 53, 69), and it is 30 times lower than the high maternal tocopherol diet dose reported to reduce hippocampus function in rodents (8). Ten days after starting the tocopherol-supplemented diet, females were mated with normal males. Three-day-old pups were suboptimally sensitized by treating with only one 50-μl ip injection (rather than two injections) of 5 μg OVA/1 mg alum (26, 28, 33). At 10, 11, and 12 days old, the pups were challenged for 15 min with 3% OVA (Fig. 1A). At 13 days old, the pups were weighed and killed, and tissues were collected. Serum was collected for analysis of IgE, liver was for tocopherol analysis, lung lavage was for leukocytes and cytokines, and lung tissue was for histology, flow cytometry, and qPCR of mediators of allergic inflammation. Liver was extracted and examined for tocopherols by high-pressure liquid chromatography with electrochemical detection as we previously described (7, 50). Bronchoalveolar lavage (BAL) cells were stained and counted as previously described (3). OVA-specific IgE was determined by ELISA as previously described (13). Lung tissue sections were fixed in cold methanol for 15 min, rehydrated for 60 min with PBS, and stained with eosin and methyl green as we previously described (6).
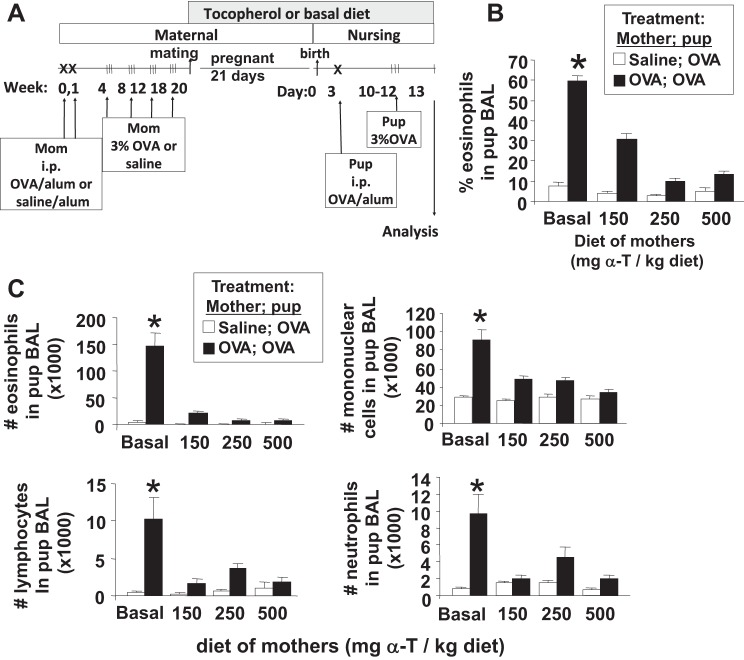
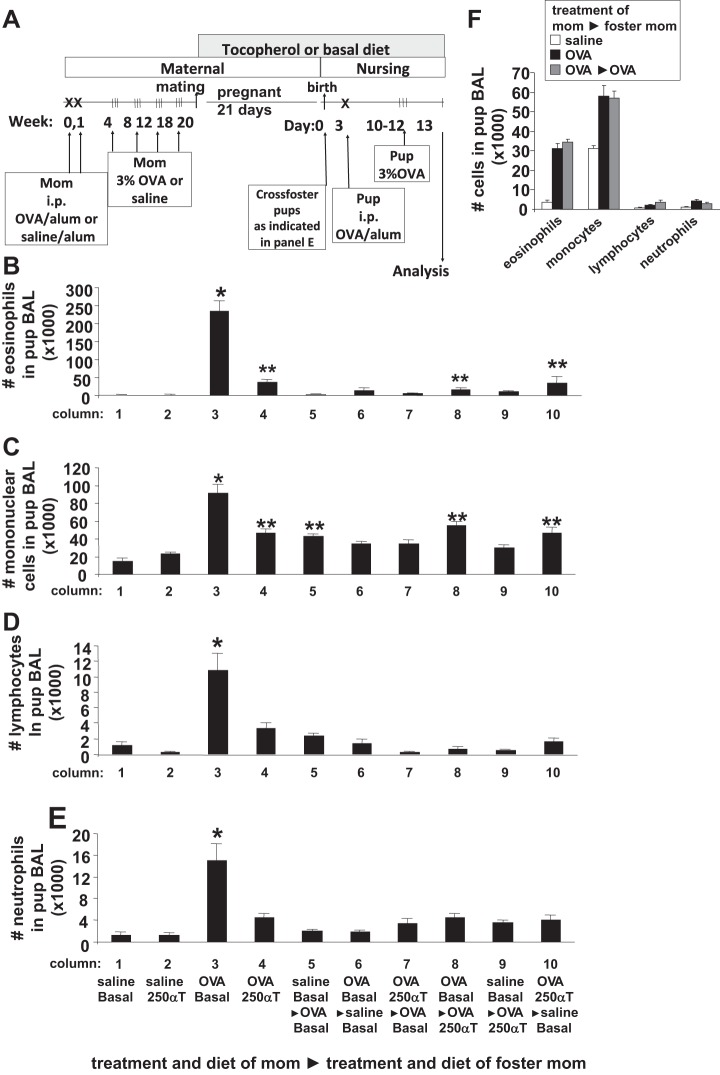
Fig. 1.
α-Tocopherol (α-T)-supplemented diet inhibited eosinophil recruitment in pups from allergic mothers. A: schematic of the timeline for d-α-tocopherol diet and ovalbumin (OVA) treatment of mothers and pups. Mothers were sensitized and challenged with OVA or saline and then, at the time of breeding, they were given diets supplemented with d-α-tocopherol. The diets contained 150, 250, or 500 mg d-α-tocopherol/kg diet. On day 3 after birth, the pups were sensitized one time with OVA/potassium aluminum sulfate (alum) by ip injection and then challenged with aerosolized 3% OVA on days 10–12. B and C: mice were treated as in A. On postnatal (PND) 13, bronchoalveolar lavage (BAL) neutrophils, eosinophils, monocytes, and lymphocytes were cytospun and counted by standard morphological criteria. n = 12 Mice/group. *P < 0.05 compared with the other groups.
Tocopherol measurement.
Diets or livers were weighed and homogenized in absolute ethanol with 5% ascorbic acid on ice. The internal standard tocol is added to each lung to determine recovery. The homogenate or plasma was extracted with an equal volume of hexane with 0.37 wt/100 wt butylated hydroxytoluene to prevent oxidation and increase recovery of tocopherol. The samples were vortexed and then centrifuged for 10 min at 9,000 g at 4°C. The hexane layer was dried under nitrogen and stored at −20°C. The samples were reconstituted in methanol, and then tocopherols were separated using a reverse-phase C18 HPLC column (Hewlett Packard) and HPLC (Waters) with 99% methanol-1% water as a mobile phase with detection with an electrochemical detector (potential 0.7 V) (Waters).
Cytokines, chemokines, and indolamine dioxygenase.
Total RNA was isolated from 50 to 100 mg lung tissue using the QIAGEN RNeasy Fibrous Tissue Mini Kit. cDNA was prepared using a SuperScript II RNase H-Reverse Transcriptase kit (Invitrogen) and analyzed by PCR on an ABI 7300 Thermal Cycler (Applied Biosystems). Taqman probes and Taqman Universal Master Mix were used as directed (Applied Biosystems). CCL24 in the BAL also was examined by ELISA (Raybiotech).
Flow cytometry analysis of pup lung, fetal liver, and cultured DCs.
Following BAL of pups, lungs were removed for analysis of dendritic cell types by flow cytometry. In the fetus, hematopoiesis occurs in the fetal livers. Gestational day 18 fetal livers were collected for analysis of fetal liver DCs. Briefly, tissues were minced and suspended in 5 ml of RPMI solution containing 1 mg/ml collagenase D (Roche) and 0.2 mg/ml DNase I (Roche) at 37°C with agitation. After 1 h, samples were filtered through sterile 70-μm mesh tissue, centrifuged, and resuspended in 5% FBS-RPMI solution. Red blood cells were lysed in 1× BD PharmLyse Lysing Buffer (BD Biosciences), and the cells were washed two times in PBS. All centrifugation steps were carried out at 300 g for 5 min. Three million cells were used per sample for immunolabeling.
For bone marrow-derived postnatal day 10 pup dendritic cell cultures, a single cell suspension of bone marrow from 10-day-old mice was placed in RPMI 1640, glutamine, HEPES, gentamycin sulfate, penicillin/streptomycin, and 10% heat-inactivated FCS. The bone marrow cells were treated with the DMSO solvent control (0.01%) or d-α-tocopherol (80 μM) as we previously described for in vitro treatment with d-α-tocopherol (7). The cells were stimulated with 5 ng granulocyte macrophage colony-stimulating factor (GM-CSF) per milliliter. The culture medium with tocopherol and GM-CSF was replaced on days 2, 4, and 7. On day 9, the cells were counted, immunolabeled with the markers described above for DCs, and examined by flow cytometry.
The cells were stained for live/dead exclusion in 500 μl PBS containing 0.25 μl of Aqua fluorescent reactive dye (Molecular Probes, Invitrogen) for 20 min at room temperature in the dark. Next, FC receptors were blocked by incubating the cells in 50 μl flow cytometry staining buffer (Biolegend) with 0.75 μl purified rat anti-mouse CD16/CD32 Mouse BD FC Block (no. 553142; BD Pharmingen) for 10 min at 4°C.
To prepare the antibodies for immunolabeling, for each sample equivalent, an antibody stock was prepared by adding the following antibodies to 50 μl of flow cytometry buffer: 1) Live/Dead Aqua fluorescent dye (no. L34957; Molecular Probes, Invitrogen), 0.25 μl/sample; 2) CD45, rat anti-mouse FITC-conjugated, 0.5 mg/ml, clone 30-F11 (no. 103107; Biolegend), 0.31 μl/sample; 3) CD11b, rat anti-mouse PE-CF594-conjugated, 0.2 mg/ml, clone M1/70 (no. 562317; BD Biosciences), 0.05 μl/sample; 4) Ly-6C, rat anti-mouse APC/Cy7-conjugated, 0.2 mg/ml, clone HK1.4 (no. 128025; Biolegend), 0.4 μl/sample; 5) CD11c, Armenian hamster anti-mouse PE/Cy7-conjugated, 0.2 mg/ml, clone N418 (no. 117317; Biolegend), 0.2 μl/sample; 6) I-A/I-E (MHCII), rat anti-mouse PerCP/Cy5.5-conjugated, 0.2 mg/ml, clone M5/114.15.2 (no. 107625; Biolegend), 0.1 μl/sample; 7) CD103, Armenian hamster anti-mouse Brilliant Violet 421-conjugated, clone 2E7 (no. 121421; Biolegend), 0.2 μl/sample; 8) CD317 (PDCA-1), rat anti-mouse APC-conjugated, 0.2 mg/ml, clone 927 (no. 127015; Biolegend), 0.5 μl/sample; 9) CD80, Armenian hamster anti-mouse PE-conjugated, 0.2 mg/ml, clone 16-10A1 (no. 104707; Biolegend), 0.5 μl/sample; and 10) CD86, rat anti-mouse Alexa Fluor 700-conjugated, 0.5 mg/ml, clone GL-1 (no. 105023; Biolegend), 0.5 μl/sample. Next, 50 μl of the antibody stock were added to the samples that were treated with FC block. The samples were incubated for 30 min at 4°C in the dark, and the cells were washed two times in 1× PBS.
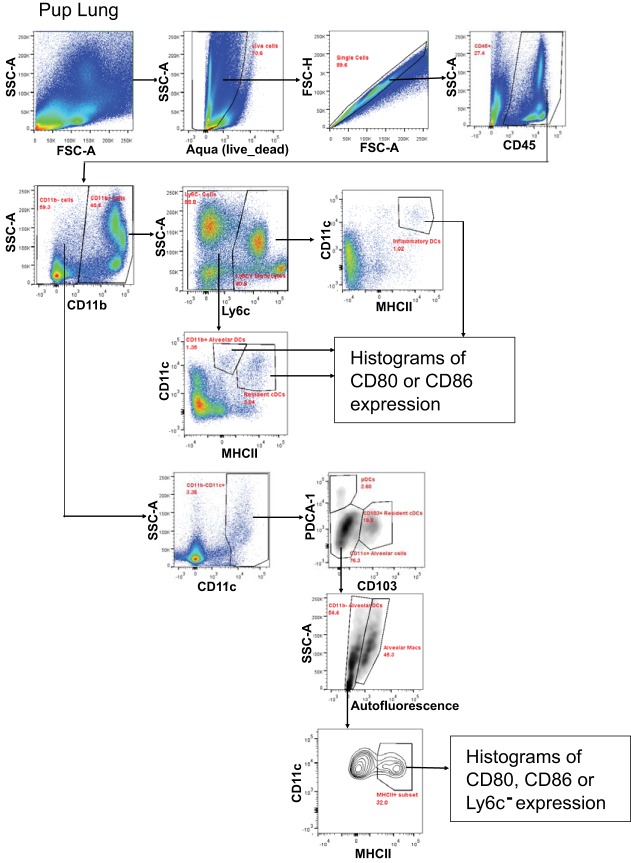
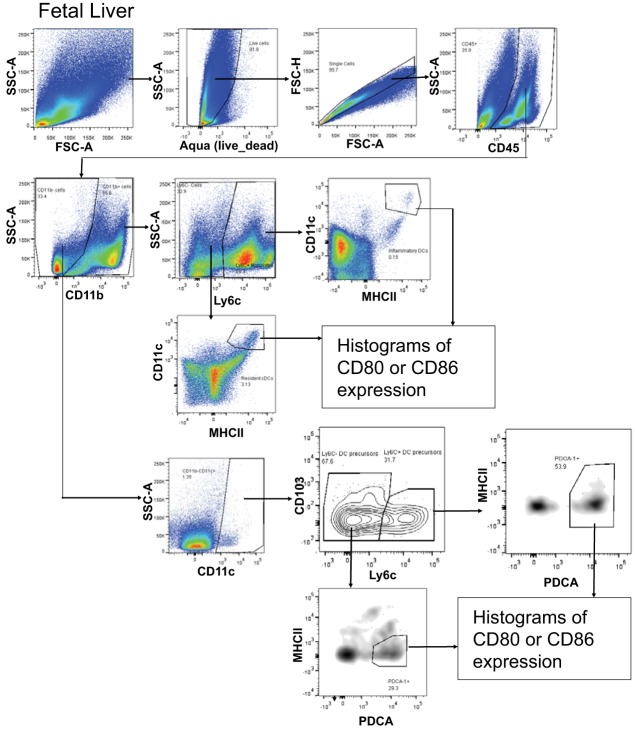
The cells were examined with a BD LSRII flow cytometer (BD Biosciences). Analysis was performed using FlowJo VX software (TreeStar). Compensation was done using FlowJo compensation wizard based on single color control staining of compensation beads (eBioscience). Nonstained controls were used to assess boundaries of live and dead populations. Only live, singlet (based on FSC-H vs. FSC-A gating), hematopoietic (CD45+) cells were used for subsequent gating of all populations. Fluorescence Minus One staining controls were used as negative controls to identify gates for populations of interest. The following subpopulations of DCs were analyzed: 1) resident conventional DCs: CD11b+Ly6C−CD11chighMHCIIhigh; 2) CD11b+ alveolar DCs: CD11b+Ly6C−CD11c+MHCII−; 3) inflammatory DCs: CD11b+Ly6C+CD11c+MHCII+; 4) plasmacytoid DCs (pDCs): CD11b−Ly6C−CD11clowPDCA-1+MHCII−; 5) resident CD103+ DCs: CD11b−Ly6C−CD11c+CD103+MHCII−; and 6) CD11b− alveolar DCs: CD11b−Ly6C−CD11c+MHCII+/−. The flow cytometry gating strategy is in Figs. 2 and 3.
Fig. 2.
Flow cytometry scheme for pup lung analysis.
Fig. 3.
Flow cytometry scheme for pup fetal liver analysis.
Statistics.
Data were analyzed by a one-way ANOVA followed by Tukey's or Dunnett's multiple-comparisons test (SigmaStat; Jandel Scientific, San Ramon, CA). Presented are the means ± SE.
RESULTS
d-α-Tocopherol supplementation of allergic female mice inhibits allergic inflammation in neonates.
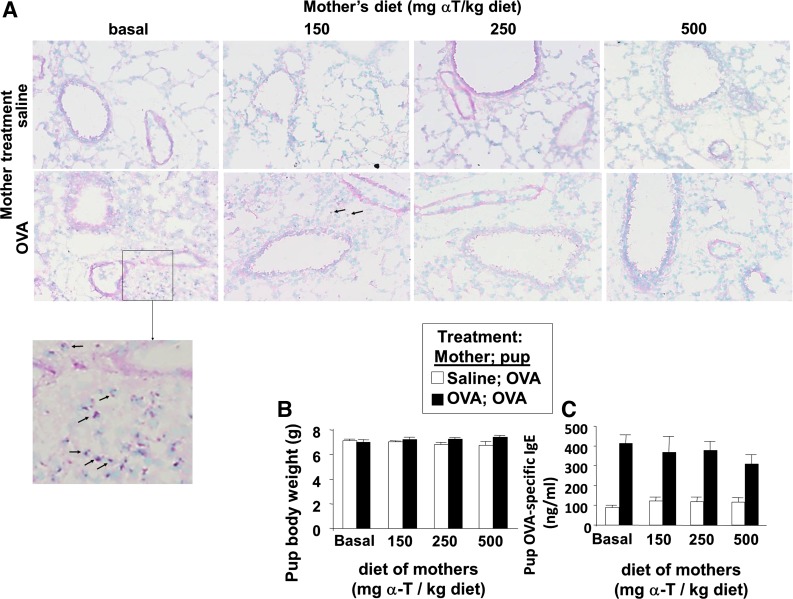
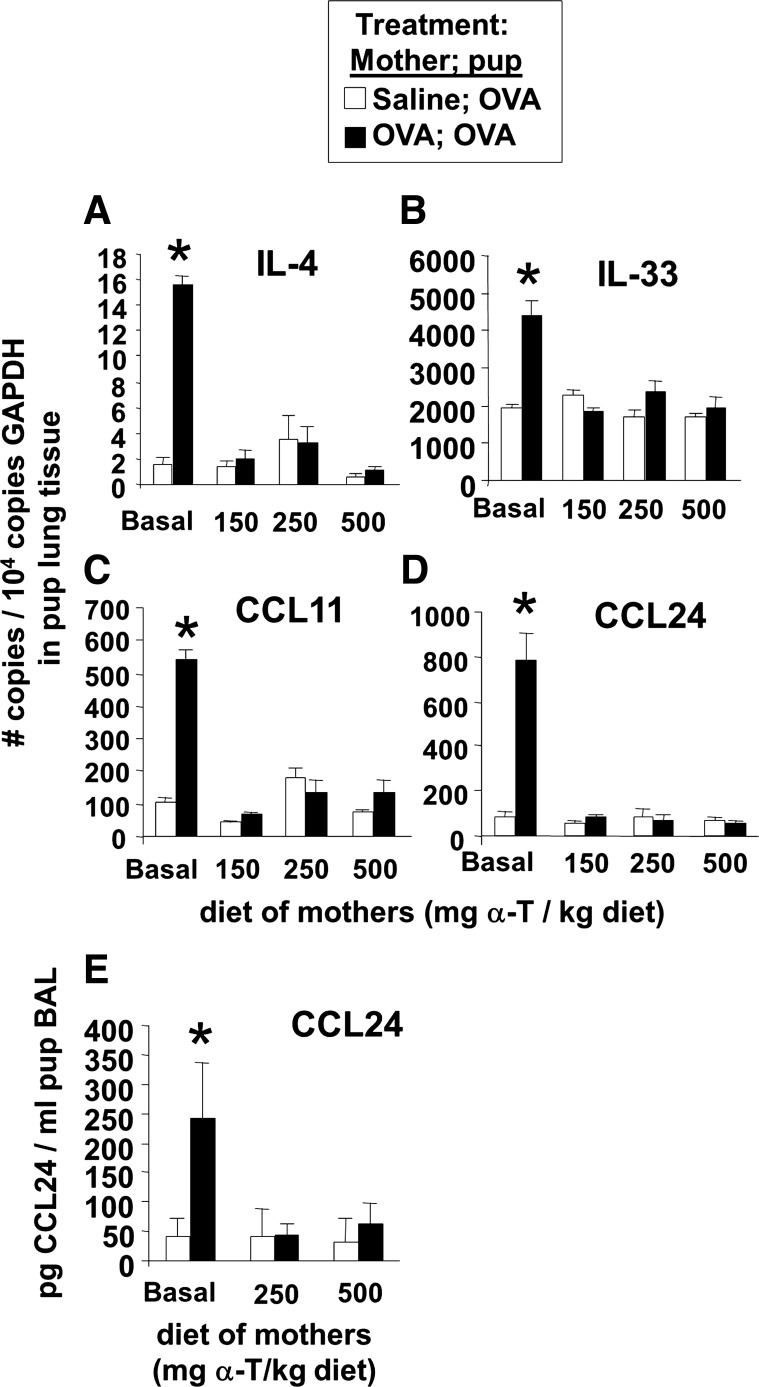
It was determined whether d-α-tocopherol supplementation of mothers during pregnancy/lactation inhibits development of allergic inflammation in their pups. In these studies, allergic responses were induced in 6-wk-old adult female mice by sensitization with OVA/alum at weeks 0 and 1 and challenge with OVA or saline three times a week during weeks 4, 8, 12, and 18 as in the timeline in Fig. 1A. Next, the females were supplemented with d-α-tocopherol at the time of mating (Fig. 1A). The mothers were given a standard basal diet (45 mg d-α-tocopherol/kg diet) or supplemented with 150, 250, or 500 mg d-α-tocopherol/kg diets (Table 1). A basal d-α-tocopherol diet was used as the control diet instead of a d-α-tocopherol-deficient diet because d-α-tocopherol is required for placenta development (37, 54). All pups from the mothers were challenged with a suboptimal regimen of OVA as previously described (46); the pups received one, instead of two, sensitizations with OVA-alum and then received three challenges with aerosolized OVA (Fig. 1A). The pups received tocopherol in utero and during lactation because it is reported that there is a contribution in utero and during nursing for the development of allergic responses in the offspring of allergic mothers (46). Compared with OVA-challenged pups from nonallergic saline-treated mothers, the OVA-treated pups from allergic mothers with basal diet had a significant increase in percent eosinophils in the BAL (Fig. 1B), an increase in total number of BAL eosinophils and monocytes (Fig. 1C), and an increase in the low numbers of BAL lymphocytes and neutrophils (Fig. 1C); this is consistent with previous reports (46). In contrast, α-tocopherol supplementation of the allergic mothers resulted in a dose-dependent inhibition of allergic inflammation in the OVA-stimulated pups from allergic mothers (Fig. 1, B and C). The 150 mg α-tocopherol/kg diet partially inhibited the percent eosinophils in the BAL, whereas the 250 and 500 mg α-tocopherol/kg diet had the greatest inhibition of the percent eosinophils in the BAL (Fig. 1B). Therefore, all subsequent studies were performed using the 250 or 500 mg α-tocopherol/kg diets. α-Tocopherol supplementation of allergic female mice also reduced eosinophils in the lung tissue of OVA-challenged pups (Fig. 4A). There was no effect of treatments on pup weight (Fig. 4B), pup numbers, or pup gender distribution (data not shown). OVA-treated pups from allergic mothers had increased serum IgE, but serum IgE was not different among the d-α-tocopherol treatment groups (Fig. 4C). Because inflammation is regulated by Th2 cytokines and chemokines, we analyzed expression of several cytokines and chemokines involved in allergic inflammation. d-α-Tocopherol supplementation of allergic female mothers inhibited OVA-induced pup lung mRNA expression of IL-4, IL-33, CCL11, and CCL24 (Fig. 5, A-D) and protein expression of CCL24 (Fig. 5E). Therefore, d-α-tocopherol supplementation of allergic mothers inhibited allergic inflammation and cytokine/chemokine mediators of allergic inflammation in OVA-challenged pups from these allergic mothers.
Fig. 4.
Maternal supplementation with d-α-tocopherol inhibited eosinophil inflammation in lung tissue of pups from allergic mothers. A: tissues were from mice in Fig. 1. Representative micrographs of perivascular regions in pup lung tissue stained with eosin and methyl green. Images were obtained with a ×20 objective on an Olympus Microscope. Arrows on the images indicate some of the eosin-labeled perivascular eosinophils. Also shown is an enlarged image of the pup lung that was from OVA-treated mothers with basal diet. B: mouse body weight. C: OVA-specific serum IgE as determined by ELISA. n = 8–10 Mice/group.
Fig. 5.
d-α-Tocopherol inhibited lung tissue cytokines and eotaxins in pups from allergic mothers. On PND13, the BAL and lung tissues were collected from mice in Fig. 1. A–D: lung tissue was placed in RNAlater and then examined by quantitative PCR for IL-4, IL-33, eotaxin 1 (CCL11), and eotaxin 2 (CCL24). E: BAL supernatant was examined for CCL24 by ELISA (Raybiotech). n = 8–10 Animals/group. *P < 0.05 compared with the corresponding saline control.
d-α-Tocopherol supplementation of the allergic mothers during pregnancy was sufficient for inhibition of allergic inflammation in cross-fostered offspring.
In Fig. 1, d-α-tocopherol supplementation was provided during pregnancy and lactation. To determine whether the maternal d-α-tocopherol supplementation during pregnancy or lactation was sufficient for inhibition of the allergic inflammation in the offspring, the neonates were cross-fostered on day 1 of birth as in the timeline in Fig. 6A. There was a contribution of the allergic mom during pregnancy and during lactation to the development of pup inflammation (Fig. 6, B–E, column 3 compared with columns 5 and 6) as previously described (44). Interestingly, cross-fostering pups from allergic mothers with 250 mg d-α-tocopherol/kg diet to allergic mothers with basal diet indicated that d-α-tocopherol supplementation of the allergic mother during pregnancy was sufficient to inhibit the development of the allergic response in the neonates (Fig. 6, B–E, column 7 compared with column 3). In addition, d-α-tocopherol supplementation during lactation reduced the allergic responses in the neonates (Fig. 6, B–E, column 8 compared with column 3). Pups from nonallergic female mice with basal diet, cross-fostered to allergic female mice with basal diet or 250 mg d-α-tocopherol/kg diet, did not develop allergic responses (Fig. 6, B–E, columns 5 and 9 compared with column 3). With basal diet, pups from allergic mothers cross-fostered to another allergic mother developed allergic responses (Fig. 6F).
Fig. 6.
d-α-Tocopherol supplementation during pregnancy was sufficient for inhibition of allergic inflammation. A: schematic of the timeline for d-α-tocopherol diet and OVA treatment of mothers and pups. Mothers were sensitized and challenged with OVA or saline and then, at the time of breeding, they were given diets supplemented with d-α-tocopherol. The diets contained 250 mg d-α-tocopherol/kg diet. On the day of birth, the pups were cross-fostered as indicated. On PND3 after birth, the pups were sensitized one time with OVA/alum by ip injection and then challenged with aerosolized 3% OVA on PND10–12. B–E: mice were treated as in A. On PND13, BAL neutrophils, eosinophils, monocytes, and lymphocytes were cytospun and counted by standard morphological criteria. Saline basal αT with the right-pointing triangle and then OVA 0αT indicates the pups were born from mothers treated with saline and fed basal α-tocopherol diet and then after the arrow, it indicates the treatments of the mother that nursed the pup for the cross-foster. n = 12 Mice/group. F: in another experiment with a set of mice separate from B–E, mice received basal diet and were treated as in A. On PND13, BAL neutrophils, eosinophils, monocytes, and lymphocytes were cytospun and counted by standard morphological criteria. Pups from allergic mothers cross-fostered to another allergic mother developed allergic responses as in pups from allergic mothers that were not cross-fostered. *P < 0.05 compared with other groups. **P < 0.05 compared with saline groups.
d-α-Tocopherol supplementation during a second pregnancy of allergic female mice inhibited development of allergic responses in the second litter of pups.
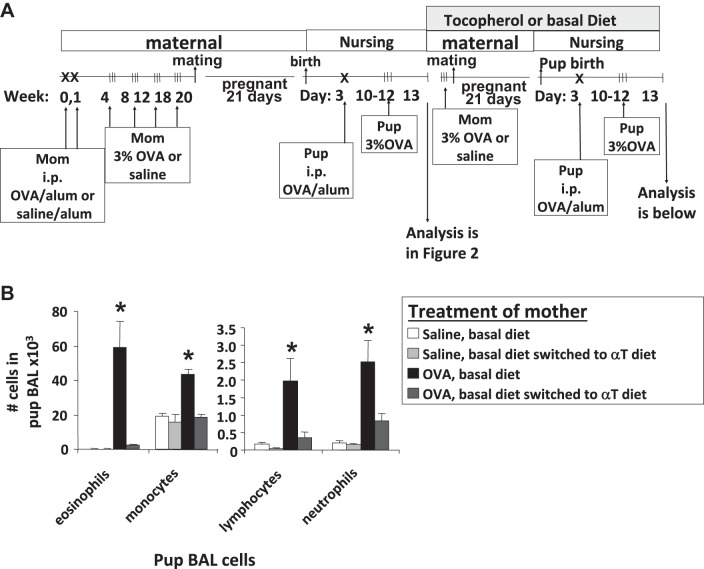
It was hypothesized that allergic moms that had a litter of allergic pups could be supplemented with d-α-tocopherol at the time of the second mating to inhibit development of allergic responses in subsequent litters of pups. Starting at the time of the second mating (timeline in Fig. 7A), the mothers, which were provided basal diet in the first mating, were provided in the second mating basal diet or switched to a diet supplemented with 500 mg d-α-tocopherol/kg diet. The offspring from allergic mothers that were supplemented with d-α-tocopherol at the time of the second mating had a >90% inhibition of BAL eosinophils in the OVA-challenged pups (Fig. 7B). Moreover, in OVA-challenged pups from allergic mothers, d-α-tocopherol reduced whole lung mRNA expression of several mediators of allergic inflammation: the cytokines IL-4, IL-33, and TSLP and the chemokines CCL11 and CCL24 (Fig. 8). At the time of collection, d-α-tocopherol had no effect on the cytokine TNF-α, the anti-inflammatory cytokine IL-10, Th1 cytokines (IL-2 and IFNγ), or MHCII in the whole lung of OVA-challenged pups from allergic mothers (Fig. 8). d-α-Tocopherol inhibition of IL-4, IL-33, and TSLP is consistent with α-tocopherol regulation of development of allergic inflammation.
Fig. 7.
d-α-Tocopherol supplementation inhibited allergic inflammation in pups from allergic mothers that previously had basal diets and litters of allergic pups. A: schematic of timeline for 500 mg α-tocopherol/kg diet and OVA treatment of mothers and pups. B: saline or OVA-treated moms were on basal diet and had a litter of allergic pups (data in Fig. 1). Next, a group of these moms were switched at time of mating to 500 mg/kg diet for the second litter of pups. Lung lavage cells from pups were cytospun, and leukocytes were counted by standard morphological criteria. n = 12 Mice/group. *P < 0.05 compared with other groups for each cell type.
Fig. 8.
Expression of cytokines, chemokines, indolamine dioxygenase (IDO), and MHCII by lungs from pups. Lung tissue from the pups in Fig. 7 was collected 24 h after the last challenge, placed in RNAlater, and then examined by qPCR. **P < 0.05 compared with all other groups. *P < 0.05 compared with the saline/basal diet group.
Because it is reported that DCs from pups of allergic moms but not control mothers transfer the risk for allergic responses to nonallergic pups (24) and because during allergic inflammation in adults lung dendritic cell expression of indolamine dioxygenase (IDO) promotes Th2 responses (83), we determined whether d-α-tocopherol supplementation regulated lung IDO expression. The OVA-challenged pups from allergic mothers from the studies in Fig. 7 had elevated whole lung expression of IDO compared with OVA-challenged pups from saline-treated mothers (Fig. 8). Interestingly, d-α-tocopherol reduced lung tissue expression of IDO in pups from allergic mothers and saline mothers (Fig. 8). These data indicate that OVA challenge induced IDO expression and/or recruitment of cells expressing IDO and that this was inhibited by the d-α-tocopherol-supplemented diet. Thus, d-α-tocopherol supplementation of mothers during a second litter blocked allergic inflammation and several cytokines, chemokines, and IDO that regulated allergic inflammation.
d-α-Tocopherol supplementation of allergic female mice reduces numbers of CD11b+ dendritic cell subsets in the fetal liver and in neonates.
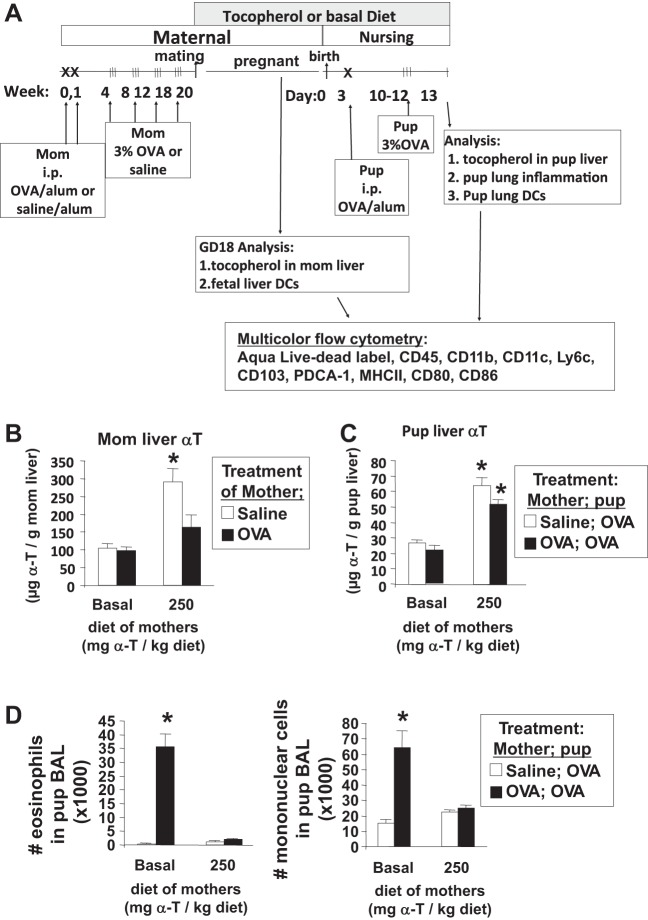
It is reported that DCs but not macrophages are critical for the development of the allergic responses in the pups from allergic mothers (24). Therefore, we determined whether d-α-tocopherol regulates the development of DCs in postnatal day 13 neonates and gestational day 18 fetuses from allergic mothers (Fig. 9A). It was also determined whether d-α-tocopherol-supplemented diets increased liver d-α-tocopherol concentrations in mothers and pups because, in the liver, d-α-tocopherol is loaded onto lipoproteins that then enter circulation for delivery to peripheral tissues. The d-α-tocopherol-supplemented diet significantly increased liver d-α-tocopherol in the saline-treated mothers threefold compared with basal diet controls (Fig. 9B). The OVA treatment reduced the d-α-tocopherol tissue concentrations in the d-α-tocopherol-supplemented mothers (Fig. 9B), which is consistent with reduced α-tocopherol levels in asthmatics (38, 39, 63, 64). Maternal d-α-tocopherol supplementation increased pup liver d-α-tocopherol 2.5-fold (Fig. 9C) and blocked allergic responses in the lung (Fig. 9D). These livers had <0.003 μg γ-tocopherol/g liver tissue for all groups (data not shown).
Fig. 9.
Analysis of liver tocopherols and dendritic cells (DCs) in the pups and mothers. A: schematic of the timeline for d-α-tocopherol diet and OVA treatment of mothers and pups. On gestational day (GD) 18, tocopherols were determined in the liver of the mothers by HPLC, and the fetal liver was analyzed for dendritic cell subsets. With another set of mothers, on PND13, the pup livers were analyzed for tocopherol isoforms by HPLC, and the pup lungs were analyzed for BAL inflammation and lung tissue dendritic cell subsets. B: mother liver α-tocopherol. *P < 0.05 compared with basal diet group. C: PND13 pup liver α-tocopherol. *P < 0.05 compared with basal diet group. D: BAL eosinophils and monocytes were determined by cytospins and cell counting. *P < 0.05 compared with other groups.
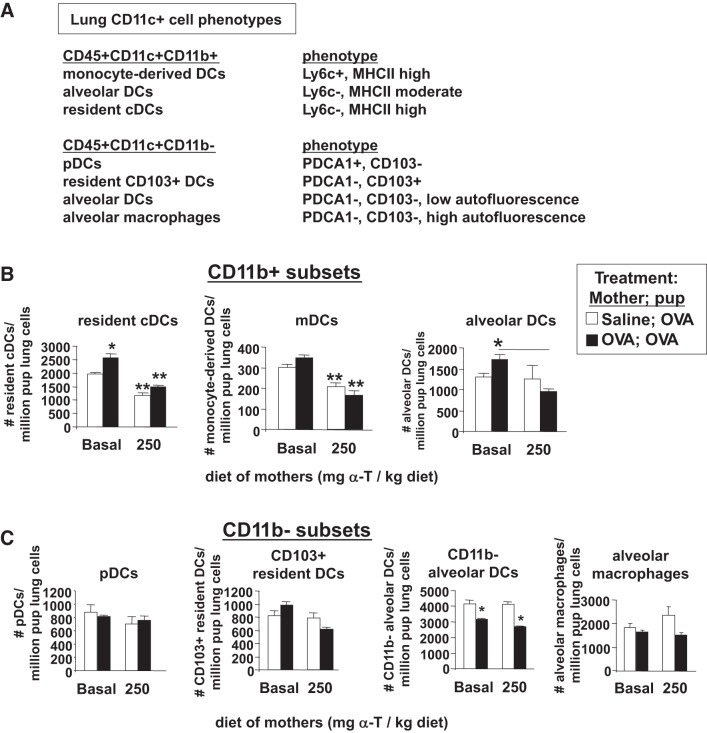
It was determined whether d-α-tocopherol regulates pup lung DCs. The cells of the postnatal day 13 neonate lungs or gestational day 18 fetal livers for the offspring outlined in Fig. 9A were dissociated and immunolabeled for cell membrane markers of lung dendritic cell subsets as previously described for the mouse lung (58). The flow cytometry analysis scheme for pup lung DCs is described in Fig. 2. There were CD11b+ and CD11b− dendritic cell subsets in the neonatal lungs, but d-α-tocopherol supplementation only altered the CD11b+ dendritic cell subsets so these two dendritic cell lineages are described here. Briefly, the following CD11b+ dendritic cell subsets were present in the postnatal day 13 OVA-challenged neonate lungs (Fig. 10A): resident DCs, monocyte-derived DCs (mDCs), and alveolar DCs. The following CD11b− cell subsets were present in the postnatal day 13, OVA-challenged neonate lungs (Fig. 10A): pDCs, resident CD103+ DCs, alveolar DCs, and alveolar macrophages. In the postnatal day 13 OVA-challenged pups, maternal supplementation with d-α-tocopherol significantly reduced the lung tissue number of CD11c+CD11b+ subsets of DCs, including resident conventional DCs, mDCs, and CD11b+ alveolar DCs (Fig. 10B). In contrast to the CD11c+CD11b+ DCs, d-α-tocopherol supplementation of the mothers did not alter the pup lung CD11c+CD11b− subsets of cells, including pDCs, resident CD103+ DCs, CD11b− alveolar DCs, and alveolar macrophages (Fig. 10C). Pups from allergic mothers had reduced numbers of CD11c+CD11b− alveolar DCs compared with pups from nonallergic mothers, but there was no effect of maternal d-α-tocopherol supplementation (Fig. 10C). For CD11c+CD11b+ DCs and CD11c+CD11b− DCs, the mean fluorescence intensity for MHCII, CD80, and CD86 was not different among the groups (data not shown).
Fig. 10.
d-α-Tocopherol supplementation of allergic female mice reduced the numbers of CD11c+CD11b+ DCs in the pup lung. The lung tissues were from the pups described in Fig. 9A. A: chart of lung CD11c+ subsets analyzed in the pup lungs. B: CD11c+CD11b+ subsets of DCs in the pup lung. The relative expression level (mean fluorescence intensity) for MHCII, CD80, and CD86 was not different among the groups (data not show). *P < 0.05 compared with the saline, basal diet group or compared with the group indicated for alveolar DCs. **P < 0.05 compared with pups from mothers with basal diets. C: CD11c+CD11b− dendritic cell subsets in the pup lung. *P < 0.05 compared with groups with saline-treated mothers. mDC, monocyte-derived DCs; pDC, plasmacytoid DCs.
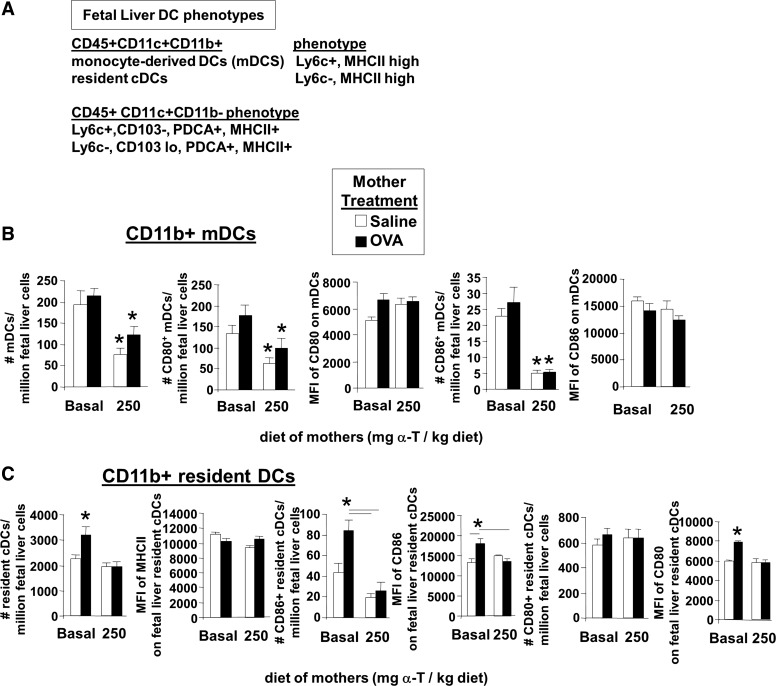
The fetal liver dendritic cell subsets were determined on gestational day 18 (Fig. 9A) because mouse fetal livers begin developing liver hematopoiesis about gestational day 16 and pups are born on gestational day 21. The fetal liver cells were immunolabeled for cell surface markers of DCs (Fig. 10A). The flow cytometry analysis scheme for fetal liver DCs is in Fig. 3. The following CD11c+CD11b+ dendritic cell subsets were present in the gestational day 18 fetal livers (Fig. 11A): mDCs and resident DCs. The following CD11c+CD11b− cell subsets were present in the gestational day 18 fetal livers (Fig. 11A): Ly6c+CD103−PDCA+MHCII+ cells and Ly6c−CD103low PDCA+MHCII+ cells. Interestingly, d-α-tocopherol supplementation of allergic mothers reduced the number of CD11c+CD11b+ DCs in gestational day 18 fetal livers, including those of the phenotype of mDCs (Fig. 11B) and phenotype of resident DCs (Fig. 11C). The CD11c+CD11b− subsets were not altered by maternal supplementation of d-α-tocopherol (data not shown).
Fig. 11.
d-α-Tocopherol supplementation of allergic female mice reduced the numbers of CD11c+CD11b+ DCs in the fetal liver. The fetal liver tissues were from the pups described in Fig. 9A. A: chart of dendritic cell subsets detected in the GD18 fetal liver. B: CD11c+CD11b+ mDCs, CD80+ CD11c+CD11b+ mDCs, and CD86+ CD11c+CD11b+ mDCs in the fetal liver and mean fluorescence intensity of CD80 and CD86 on these fetal liver DCs. *P < 0.05 compared with groups with basal diet. C: CD11c+CD11b− resident DCs, CD80+CD11c+CD11b− resident DCs, and CD86+CD11c+CD11b− resident DCs in the fetal liver and mean fluorescence intensity of CD80 and CD86 on these fetal liver DCs. *P < 0.05 compared with the other groups or compared with the groups indicated. MFI, mean fluorescence intensity.
To determine whether d-α-tocopherol can directly regulate differentiation of DCs, we determined whether supplementation in vitro with d-α-tocopherol blocked development of bone marrow-derived CD11b+ DCs. For these studies, bone marrow from 10-day-old neonates from mothers with basal diet was incubated with GM-CSF for 8 days in vitro in the presence of d-α-tocopherol or the solvent control DMSO. d-α-Tocopherol reduced the number of CD45+ CD11b+CD11+ DCs and the number of cells with resident DC phenotype (CD45+CD11b+CD11c+Ly6c−MHCII− DCs) (Fig. 12) without affecting the percent of viable cells in the culture (data not shown). In summary, d-α-tocopherol reduced the number of CD11b+CD11c+ DCs in vitro, in the fetal liver and in the neonate.
Fig. 12.
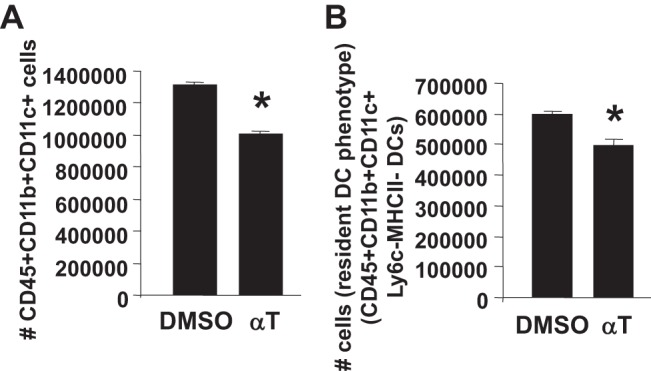
d-α-Tocopherol inhibited the generation of bone marrow-derived CD11c+CD11b+ DCs in vitro. Bone marrow from PND10 pups was cultured for 10 days with GM-CSF in the presence of 0.01% DMSO (solvent control) or 80 μM d-α-tocopherol (as we previously described for in vitro cell loading with d-α-tocopherol) (7). A: no. of CD45+CD11c+CD11b+ cells. B: no. of cells with resident dendritic cell phenotype (CD45+CD11b+CD11c+Ly6c−MHCII−). There was no difference in the %live cells between the two groups. There were no cells from the culture with the monocyte-derived phenotype (CD45+CD11c+CD11b+Ly6c+, MHCII high) (data not shown). n = 5–6 from a representative experiment of two experiments. *P < 0.05 compared with the DMSO-treated control.
DISCUSSION
In this report, the development of allergic inflammation in pups from allergic mothers was inhibited by supplementation of the mother with d-α-tocopherol during pregnancy and lactation. In the pups, there was a reduction in mediators of allergic inflammation, including IL-4, IL-33, TSLP, CCL11, and CCL24. In addition, after allergic mothers had a first litter of pups that responded to antigen challenge, d-α-tocopherol supplementation of these mothers during the second pregnancy prevented development of allergic inflammation in the second litter of pups that were challenged with antigen. The studies with cross-fostering at birth demonstrated that administration of d-α-tocopherol during pregnancy or during lactation was sufficient to inhibit development of allergic inflammation in the pups. d-α-Tocopherol did not affect body weight or lung weight, which is consistent with previous reports for d-α-tocopherol (7, 50, 52). d-α-Tocopherol supplementation of the mothers inhibited generation of CD11c+CD11b+ DCs in the fetal liver and in antigen-challenged pup lungs. d-α-Tocopherol has, at least, a direct effect on dendritic cell development because d-α-tocopherol inhibited generation of CD11c+CD11b+ bone marrow-derived DCs in vitro. In contrast, d-α-tocopherol did not alter CD11c+CD11b− DCs in vivo or in vitro, indicating specificity for d-α-tocopherol regulation of dendritic cell differentiation to CD11c+CD11b+ DCs. There was no effect of d-α-tocopherol on the level of expression of MHCII, CD80, or CD86 by DCs. This is the first report that maternal d-α-tocopherol supplementation of allergic mothers reduces development of allergic responses in offspring and regulates numbers of select dendritic cell subsets. These results have important implications for vitamin E regulation of the development of allergic disease in future generations.
The prevalence of allergic diseases has dramatically increased in the last 40 years (27, 73, 76), suggesting that there are changes in environmental factors. Allergic diseases originate as complex environmental and genetic interactions that may start early in life (49), a time when the airways and the immune system are developing. It is suggested that in utero and early exposures to environmental factors are critical for risk of allergic disease (9). In reports examining human maternal and paternal asthma associations with development of allergies in offspring, most associations are with maternal asthma (46). In animal studies, allergen challenge of the mother predisposes the offspring to responses to suboptimal doses of allergens and, as in humans (46), the offspring's responses are not specific to the allergen that the mother had been exposed (26, 28–30, 32, 33, 36). The sensitization to allergens and allergic responses are dependent on DCs, which produce regulatory cytokines (72, 80). Interestingly, DCs, but not macrophages, from offspring of allergic mothers transfer the risk for allergies to naïve neonates, indicating a functional change in neonatal DCs during development (24). Maternal exposure to environmental factors, including high-fat diets, can alter neonatal hematopoietic or metabolic function (14, 25, 35, 47, 55, 61, 75, 82). In our studies, maternal supplementation with d-α-tocopherol inhibited development of CD11c+CD11b+ DCs in the fetus and in neonates.
Dietary tocopherols are taken up from the intestine (62) and transported through the lymph to the blood and then to the liver. In the liver, α-tocopherol is preferentially transferred, over other tocopherol isoforms, to lipoproteins by the liver αTTP (11, 45, 59, 68, 81). αTTP is also expressed by trophoblast, fetal endothelium, and amnion epithelium of the placenta (54). αTTP transfers α-tocopherol to low-density lipoprotein (LDL) and high-density lipoprotein (HDL) particles that then enter the blood (23). LDL or HDL with tocopherols are taken up by cells by plasma phospholipid transfer protein, scavenger receptors, or the lipoprotein lipase pathway (21). It is reported that basal levels of α-tocopherol are required for placentation (37, 54). In our studies, maternal supplementation with d-α-tocopherol raised the maternal liver α-tocopherol level 3-fold and the pup liver α-tocopherol 2.5-fold, consistent with the fold tocopherol changes in human and mouse tissues after supplementation (2, 7, 19, 20, 50, 51). This increase in maternal d-α-tocopherol inhibited allergic responses in neonates and inhibited development of CD11c+CD11b+ dendritic cell subsets in utero and in the neonate.
The numbers of neonatal lung dendritic cell subsets in our studies were consistent with other reports of proportions of DCs in lungs of neonatal mice and adult mice. Reports indicate that, for neonatal mice, there are about 1 × 105 CD11c+MHCII+ cells/pup lung in 4- to 11-day-old neonates (85), and there are about 5 × 104 pDCs/pup lung in 15-day-old neonates (4). Consistent with this, in our studies in which there were 20 × 106 lung cells/10-day-old C57BL/6 pup lung (data not shown), there were 2 × 105 CD11c+ cells/pup lung and 2 × 104 pDCs/pup lung. In addition, the proportions of several dendritic cell subsets in pups in our studies were similar to reports of adult lungs. Briefly, the ratio of pDC/cDC is reported as about 0.2–0.3 in the OVA-challenged adult mouse lung (41). In the 10-day-old pups in our studies, the lung pDC-to-cDC ratio was also 0.3 in the lungs of OVA-challenged pups from allergic mothers with basal tocopherol diet. Interestingly, with d-α-tocopherol supplementation of allergic mothers, there was a decrease in numbers of pup lung cDC, resulting in an increase in the pDC-to-cDC ratio to 0.53. Also in adult mice, about 8% of lung CD11c+ cells are pDCs (60) and 9% of lung CD11c+ DCs are CD103+ (57). Likewise, in the 10-day-old C57BL/6 pup lungs in our study, 8% of lung CD11c+ cells were pDCs and 9.7% of CD11c+ DCs were CD103+. Thus, proportions of DCs in the neonatal mouse lungs in our studies were similar to the few reports for the neonatal mouse lung and to reports for the adult mouse lung.
In our studies, maternal d-α-tocopherol supplementation decreased the number of pup lung CD11b+ dendritic cell subsets but not the number of pup lung CD11b− dendritic cell subsets. This specificity of α-tocopherol regulation of the CD11b+ dendritic cell subsets suggests that tocopherols may regulate signals for dendritic cell differentiation of this DC subset or regulate signals for expression of CD11b. The generation of CD11b+ DCs is regulated by signals and several transcription factors that bind the CD11b promoter (74). Some signals that regulate expression of CD11b on DCs include GM-CSF (86), ERK5 (78), aldehyde dehydrogenase (86), and retinoic acid (5, 40, 66, 67, 86). The CD11b promoter has binding sites for the transcription factors Sp1, Vav1/PU.1, ets, AP-2, and RAR (12, 31). Whether α-tocopherol regulates GM-CSF signals or regulates transcription factors that activate the CD11b promoter in DCs is not known and is under investigation.
In DCs, signaling through protein kinase C and NF-kB induces IDO expression (56). The IDO pathway can function to inhibit Th1 responses (84), whereas IDO has an opposing role in which it increases Th2 immune responses (84). Consistent with this, IDO-deficient adult mice have fewer mature DCs in draining lymph nodes, reduced OVA-induced inflammation, and lower airway hyperresponsiveness (83). It is reported that, in patients, serum IDO is increased during seasonal allergen exposure and that IDO expression is induced by the high-affinity receptor for IgE, FcϵRI (77). It is also reported that, during pregnancy, IDO promotes tolerance toward the fetus (43). In our studies, pups from allergic mothers had elevated OVA-induced lung IDO expression, and this was reduced by maternal d-α-tocopherol supplementation without affecting the number of pups or gender distribution. Thus the OVA challenge induced lung IDO expression and/or recruitment of cells expressing IDO in the pup lung, and this was inhibited by maternal supplementation with d-α-tocopherol.
Reports conflict as to whether tocopherols modulate inflammation. Most clinical studies on vitamin E include mixed forms of natural and synthetic tocopherols from supplementation or diet. We recently demonstrated in adult mice that γ-tocopherol increases allergic responses and that α-tocopherol inhibits allergic responses (7, 19). We also recently reported in a study with 4,500 individuals that α-tocopherol associates positively with adult human lung spirometry [forced expiratory volume in 1 s (FEV1)] and γ-tocopherol associates negatively with human lung spirometry (FEV1), with high levels of γ-tocopherol associating with up to 300 ml lower FEV1 (48). The opposing regulatory effects of these two tocopherol isoforms have implications on interpretation of clinical studies with mixed tocopherols, and we have written reviews discussing these implications (1, 15, 17, 19, 20). In addition, α-tocopherol levels are low in asthmatics (38, 39, 63, 64) and consistent with this, in our studies, OVA challenge reduced mother α-tocopherol tissue concentrations (Fig. 9B). Therefore, because α-tocopherol levels are low in asthmatics (38, 39, 63, 64) and because α-tocopherol can reduce inflammation, an increase in α-tocopherol in the presence of low γ-tocopherol may be beneficial in limiting development of allergic disease in offspring. Maternal exposure to other environmental factors, including high-fat diets, can alter neonatal hematopoietic or metabolic function (14, 25, 35, 47, 55, 61, 75, 82). Our studies indicate that d-α-tocopherol inhibits the generation of allergic disease upon exposure to allergen and reduces hematopoiesis of CD11c+CD11b+ DCs but not CD11c+CD11b− dendritic cell subsets. Maternal α-tocopherol supplementation did not completely reduce the dendritic cell subsets expressing CD11b and thus this may maintain CD11c+CD11b+ dendritic cell immunity that would be critical for nonallergic immune defense.
In summary, we identified novel regulatory functions for d-α-tocopherol. Maternal supplementation with d-α-tocopherol decreased allergic responses in offspring from allergic mothers and decreased development of CD11c+CD11b+ dendritic cell types in utero and in antigen-challenged neonate lungs. Moreover, there was specificity of regulation of DCs by d-α-tocopherol because d-α-tocopherol reduced the number of CD11c+CD11b+ but not CD11c+CD11b− dendritic cell types. These studies of d-α-tocopherol regulation of inflammation provide a basis toward designing drugs, supplements, and diets that more effectively modulate these pathways in allergic disease. Information from our studies will have significant impact on interpretation of vitamin E clinical studies, on the design of future clinical studies with vitamin E, and on our understanding of vitamin E regulation of dendritic cell function during allergic inflammation. Moreover, these studies may have significant implications for dietary impact on risk for allergic disease in future generations.
GRANTS
These studies were supported by National Institutes of Health Grants R01-AT-004837 (J. M. Cook-Mills) and R01-HLB-111624 (J. M. Cook-Mills).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
H.A.-V., S.B., F.S., and J.M.C.-M. performed experiments; H.A.-V., S.B., F.S., and J.M.C.-M. analyzed data; H.A.-V., S.B., F.S., and J.M.C.-M. interpreted results of experiments; H.A.-V., S.B., F.S., and J.M.C.-M. approved final version of manuscript; J.M.C.-M. conception and design of research; J.M.C.-M. prepared figures; J.M.C.-M. drafted manuscript; J.M.C.-M. edited and revised manuscript.
REFERENCES
- 1.Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Vitamin E isoforms as modulators of lung inflammation. Nutrients 5: 4347–4363, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Vitamin E isoforms differentially regulate intercellular adhesion molecule-1 activation of PKCalpha in human microvascular endothelial cells. PLoS ONE 7: e41054, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdala-Valencia H, Earwood J, Bansal S, Jansen M, Babcock G, Garvy B, Wills-Karp M, Cook-Mills JM. Nonhematopoietic NADPH oxidase regulation of lung eosinophilia and airway hyperresponsiveness in experimentally induced asthma. Am J Physiol Lung Cell Mol Physiol 292: L1111–L1125, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Garawi A, Fattouh R, Botelho F, Walker TD, Goncharova S, Moore CL, Mori M, Erjefalt JS, Chu DK, Humbles AA, Kolbeck R, Stampfli MR, O'Byrne PM, Coyle AJ, Jordana M. Influenza A facilitates sensitization to house dust mite in infant mice leading to an asthma phenotype in adulthood. Mucosal Immunol 4: 682–694, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Beijer MR, Kraal G, den Haan JM. Vitamin A and dendritic cell differentiation. Immunology 142: 39–45, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berdnikovs S, Abdala-Valencia H, Cook-Mills JM. Endothelial cell PTP1B regulates leukocyte recruitment during allergic inflammation. Am J Physiol Lung Cell Mol Physiol 304: L240–L249, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, Garcia A, Bryce P, Cook-Mills J. Isoforms of vitamin E have opposing immunoregulatory funcitons during inflammation by regulating leukocyte recruitment. J Immunol 182: 4395–4405, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betti M, Ambrogini P, Minelli A, Floridi A, Lattanzi D, Ciuffoli S, Bucherelli C, Prospero E, Frontini A, Santarelli L, Baldi E, Benetti F, Galli F, Cuppini R. Maternal dietary loads of alpha-tocopherol depress protein kinase C signaling and synaptic plasticity in rat postnatal developing hippocampus and promote permanent deficits in adult offspring. J Nutr Biochem 22: 60–70, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Bousquet J, Anto J, Auffray C, Akdis M, Cambon-Thomsen A, Keil T, Haahtela T, Lambrecht BN, Postma DS, Sunyer J, Valenta R, Akdis CA, Annesi-Maesano I, Arno A, Bachert C, Ballester F, Basagana X, Baumgartner U, Bindslev-Jensen C, Brunekreef B, Carlsen KH, Chatzi L, Crameri R, Eveno E, Forastiere F, Garcia-Aymerich J, Guerra S, Hammad H, Heinrich J, Hirsch D, Jacquemin B, Kauffmann F, Kerkhof M, Kogevinas M, Koppelman GH, Kowalski ML, Lau S, Lodrup-Carlsen KC, Lopez-Botet M, Lotvall J, Lupinek C, Maier D, Makela MJ, Martinez FD, Mestres J, Momas I, Nawijn MC, Neubauer A, Oddie S, Palkonen S, Pin I, Pison C, Rance F, Reitamo S, Rial-Sebbag E, Salapatas M, Siroux V, Smagghe D, Torrent M, Toskala E, van Cauwenberge P, van Oosterhout AJ, Varraso R, von Hertzen L, Wickman M, Wijmenga C, Worm M, Wright J, Zuberbier T. MeDALL (Mechanisms of the Development of ALLergy): an integrated approach from phenotypes to systems medicine. Allergy 66: 596–604, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Bousquet J, Bousquet PJ, Godard P, Daures JP. The public health implications of asthma. Bull World Health Organ 83: 548–554, 2005 [PMC free article] [PubMed] [Google Scholar]
- 11.Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. FASEB J 13: 1145–1155, 1999 [PubMed] [Google Scholar]
- 12.Brugnoli F, Lambertini E, Varin-Blank N, Piva R, Marchisio M, Grassilli S, Miscia S, Capitani S, Bertagnolo V. Vav1 and PU.1 are recruited to the CD11b promoter in APL-derived promyelocytes: role of Vav1 in modulating PU1-containing complexes during ATRA-induced differentiation. Exp Cell Res 316: 38–47, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Bryce PJ, Geha R, Oettgen HC. Desloratadine inhibits allergen-induced airway inflammation and bronchial hyperresponsiveness and alters T-cell responses in murine models of asthma. J Allergy Clin Immunol 112: 149–158, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Burke KT, Colvin PL, Myatt L, Graf GA, Schroeder F, Woollett LA. Transport of maternal cholesterol to the fetus is affected by maternal plasma cholesterol concentrations in the golden Syrian hamster. J Lipid Res 50: 1146–1155, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook-Mills JM. Eosinophil-endothelial cell interactions. Eosinophils in health and disease. In: Eosinophils in Health and Disease, edited by Lee JJaR. New York, NY: Elsevier, 2012, p. 139–153 [Google Scholar]
- 16.Cook-Mills JM. Isoforms of vitamin E differentially regulate PKC alpha and inflammation: a review. J Clin Cell Immunol 4: 1000137, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook-Mills JM, Abdala-Valencia H, Hartert T. Two faces of vitamin e in the lung. Am J Respir Crit Care Med 188: 279–284, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook-Mills JM, Marchese M, Abdala-Valencia H. Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal 15: 1607–1638, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cook-Mills JM, McCary CA. Isoforms of vitamin E differentially regulate inflammation. Endocr Metab Immune Disord Drug Targets 10: 348–366, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desrumaux C, Deckert V, Athias A, Masson D, Lizard G, Palleau V, Gambert P, Lagrost L. Plasma phospholipid transfer protein prevents vascular endothelium dysfunction by delivering alpha-tocopherol to endothelial cells. FASEB J 13: 883–892, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Dow L, Tracey M, Villar A, Coggon D, Margetts BM, Campbell MJ, Holgate ST. Does dietary intake of vitamins C and E influence lung function in older people? Am J Respir Crit Care Med 154: 1401–1404, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Fechner H, Schlame M, Guthmann F, Stevens PA, Rustow B. alpha- and delta-tocopherol induce expression of hepatic alpha-tocopherol-transfer-protein mRNA. Biochem J 331: 577–581, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fedulov AV, Kobzik L. Allergy risk is mediated by dendritic cells with congenital epigenetic changes. Am J Respir Cell Mol Biol 44: 285–292, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fedulov AV, Leme A, Yang Z, Dahl M, Lim R, Mariani TJ, Kobzik L. Pulmonary exposure to particles during pregnancy causes increased neonatal asthma susceptibility. Am J Respir Cell Mol Biol 38: 57–67, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fedulov AV, Leme AS, Kobzik L. Duration of allergic susceptibility in maternal transmission of asthma risk. Am J Reprod Immunol 58: 120–128, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Friebele E. The attack of asthma. Environ Health Perspect 104: 22–25, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamada K, Suzaki Y, Goldman A, Ning YY, Goldsmith C, Palecanda A, Coull B, Hubeau C, Kobzik L. Allergen-independent maternal transmission of asthma susceptibility. J Immunol 170: 1683–1689, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Herz U, Joachim R, Ahrens B, Scheffold A, Radbruch A, Renz H. Allergic sensitization and allergen exposure during pregnancy favor the development of atopy in the neonate. Int Arch Allergy Immunol 124: 193–196, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Herz U, Joachim R, Ahrens B, Scheffold A, Radbruch A, Renz H. Prenatal sensitization in a mouse model. Am J Respir Crit Care Med 162: S62–65, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Hickstein DD, Baker DM, Gollahon KA, Back AL. Identification of the promoter of the myelomonocytic leukocyte integrin CD11b. Proc Natl Acad Sci USA 89: 2105–2109, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hubeau C, Apostolou I, Kobzik L. Adoptively transferred allergen-specific T cells cause maternal transmission of asthma risk. Am J Pathol 168: 1931–1939, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hubeau C, Apostolou I, Kobzik L. Targeting of CD25 and glucocorticoid-induced TNF receptor family-related gene-expressing T cells differentially modulates asthma risk in offspring of asthmatic and normal mother mice. J Immunol 178: 1477–1487, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Hunter SC, Cahoon EB. Enhancing vitamin E in oilseeds: unraveling tocopherol and tocotrienol biosynthesis. Lipids 42: 97–108, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Izzotti A, Balansky RM, Cartiglia C, Camoirano A, Longobardi M, De Flora S. Genomic and transcriptional alterations in mouse fetus liver after transplacental exposure to cigarette smoke. FASEB J 17: 1127–1129, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Jarrett E, Hall E. Selective suppression of IgE antibody responsiveness by maternal influence. Nature 280: 145–147, 1979 [DOI] [PubMed] [Google Scholar]
- 37.Jishage K, Tachibe T, Ito T, Shibata N, Suzuki S, Mori T, Hani T, Arai H, Suzuki H. Vitamin E is essential for mouse placentation but not for embryonic development itself. Biol Reprod 73: 983–987, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Kalayci O, Besler T, Kilinc K, Sekerel BE, Saraclar Y. Serum levels of antioxidant vitamins (alpha tocopherol, beta carotene, and ascorbic acid) in children with bronchial asthma. Turk J Peds 42: 17–21, 2000 [PubMed] [Google Scholar]
- 39.Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. Altered lung antioxidant status in patients with mild asthma. Lancet 354: 482–483, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Klebanoff CA, Spencer SP, Torabi-Parizi P, Grainger JR, Roychoudhuri R, Ji Y, Sukumar M, Muranski P, Scott CD, Hall JA, Ferreyra GA, Leonardi AJ, Borman ZA, Wang J, Palmer DC, Wilhelm C, Cai R, Sun J, Napoli JL, Danner RL, Gattinoni L, Belkaid Y, Restifo NP. Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. J Exp Med 210: 1961–1976, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kool M, van Nimwegen M, Willart MA, Muskens F, Boon L, Smit JJ, Coyle A, Clausen BE, Hoogsteden HC, Lambrecht BN, Hammad H. An anti-inflammatory role for plasmacytoid dendritic cells in allergic airway inflammation. J Immunol 183: 1074–1082, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Lass A, Forster MJ, Sohal RS. Effects of coenzyme Q10 and alpha-tocopherol administration on their tissue levels in the mouse: elevation of mitochondrial alpha-tocopherol by coenzyme Q10. Free Rad Biol Med 26: 1375–1382, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Le AV, Broide DH. Indoleamine-2,3-dioxygenase modulation of allergic immune responses. Curr Allergy Asthma Rep 6: 27–31, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Leme AS, Hubeau C, Xiang Y, Goldman A, Hamada K, Suzaki Y, Kobzik L. Role of breast milk in a mouse model of maternal transmission of asthma susceptibility. J Immunol 176: 762–769, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Leonard SW, Terasawa Y, Farese RV, Jr, Traber MG. Incorporation of deuterated RRR- or all-rac-alpha-tocopherol in plasma and tissues of alpha-tocopherol transfer protein–null mice. Am J Clin Nutr 75: 555–560, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Lim RH, Kobzik L. Maternal transmission of asthma risk. Am J Reprod Immunol 61: 1–10, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Lim RH, Kobzik L, Dahl M. Risk for asthma in offspring of asthmatic mothers versus fathers: a meta-analysis. PLoS ONE 5: e10134, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marchese ME, Kumar R, Colangelo LA, Avila PC, Jacobs DR, Jr, Gross M, Sood A, Liu K, Cook-Mills JM. The vitamin E isoforms alpha-tocopherol and gamma-tocopherol have opposite associations with spirometric parameters: the CARDIA study. Respir Res 15: 31, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinez FD. Genes, environments, development and asthma: a reappraisal. Eur Respir J 29: 179–184, 2007 [DOI] [PubMed] [Google Scholar]
- 50.McCary CA, Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Supplemental and highly elevated tocopherol doses differentially regulate allergic inflammation: reversibility of alpha-tocopherol and gamma-tocopherol's effects. J Immunol 186: 3674–3685, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCary CA, Yoon Y, Panagabko C, Cho W, Atkinson J, Cook-Mills JM. Vitamin E isoforms directly bind PKCalpha and differentially regulate activation of PKCalpha. Biochem J 441: 189–198, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meydani SN, Shapiro AC, Meydani M, Macauley JB, Blumberg JB. Effect of age and dietary fat (fish, corn and coconut oils) on tocopherol status of C57BL/6Nia mice. Lipids 22: 345–350, 1987 [DOI] [PubMed] [Google Scholar]
- 53.Meydani SN, Yogeeswaran G, Liu S, Baskar S, Meydani M. Fish oil and tocopherol-induced changes in natural killer cell-mediated cytotoxicity and PGE2 synthesis in young and old mice. J Nutr 118: 1245–1252, 1988 [DOI] [PubMed] [Google Scholar]
- 54.Muller-Schmehl K, Beninde J, Finckh B, Florian S, Dudenhausen JW, Brigelius-Flohe R, Schuelke M. Localization of alpha-tocopherol transfer protein in trophoblast, fetal capillaries' endothelium and amnion epithelium of human term placenta. Free Radic Res 38: 413–420, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Odaka Y, Nakano M, Tanaka T, Kaburagi T, Yoshino H, Sato-Mito N, Sato K. The influence of a high-fat dietary environment in the fetal period on postnatal metabolic and immune function. Obesity 18: 1688–1694, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Ogasawara N, Oguro T, Sakabe T, Matsushima M, Takikawa O, Isobe K, Nagase F. Hemoglobin induces the expression of indoleamine 2,3-dioxygenase in dendritic cells through the activation of PI3K, PKC, and NF-kappaB and the generation of reactive oxygen species. J Cell Biochem 108: 716–725, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B, Hammad H, Lambrecht BN. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 38: 322–335, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Plantinga M, Hammad H, Lambrecht BN. Origin and functional specializations of DC subsets in the lung. Eur J Immunol 40: 2112–2118, 2010 [DOI] [PubMed] [Google Scholar]
- 59.Podda M, Weber C, Traber MG, Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J Lipid Res 37: 893–901, 1996 [PubMed] [Google Scholar]
- 60.Raymond M, Rubio M, Fortin G, Shalaby KH, Hammad H, Lambrecht BN, Sarfati M. Selective control of SIRP-alpha-positive airway dendritic cell trafficking through CD47 is critical for the development of T(H)2-mediated allergic inflammation. J Allergy Clin Immunol 124: 1333–1342, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Rebholz SL, Jones T, Burke KT, Jaeschke A, Tso P, D'Alessio DA, Woollett LA. Multiparity leads to obesity and inflammation in mothers and obesity in male offspring. Am J Physiol Endocrinol Metab 302: E449–E457, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reboul E, Klein A, Bietrix F, Gleize B, Malezet-Desmoulins C, Schneider M, Margotat A, Lagrost L, Collet X, Borel P. Scavenger receptor class B type I (SR-BI) is involved in vitamin E transport across the enterocyte. J Biol Chem 281: 4739–4745, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schunemann HJ, Grant BJ, Freudenheim JL, Muti P, Browne RW, Drake JA, Klocke RA, Trevisan M. The relation of serum levels of antioxidant vitamins C and E, retinol and carotenoids with pulmonary function in the general population. Am J Respir Crit Care Med 163: 1246–1255, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Shvedova AA, Kisin ER, Kagan VE, Karol MH. Increased lipid peroxidation and decreased antioxidants in lungs of guinea pigs following an allergic pulmonary response. Tox Appl Pharm 132: 72–81, 1995 [DOI] [PubMed] [Google Scholar]
- 65.Smit HA, Grievink L, Tabak C. Dietary influences on chronic obstructive lung disease and asthma: a review of the epidemiological evidence. Proc Nutr Soc 58: 309–319, 1999 [DOI] [PubMed] [Google Scholar]
- 66.Stock AT, Bedoui S. Vitamin A notches up CD11b hi DC development. Eur J Immunol 43: 1441–1444, 2013 [DOI] [PubMed] [Google Scholar]
- 67.Tang H, Chen F, Tan Q, Tan S, Liu L, Zhang F. Regulation of CD11b transcription by decreasing PRC2 and increased acH4 level during ATRA-induced HL-60 differentiation. Acta Biochim Biophys Sin (Shanghai) 41: 588–593, 2009 [DOI] [PubMed] [Google Scholar]
- 68.Traber MG, Kayden HJ. Preferential incorporation of alpha-tocopherol vs gamma-tocopherol in human lipoproteins. Am J Clin Nutr 49: 517–526, 1989 [DOI] [PubMed] [Google Scholar]
- 69.Traber MG, Siddens LK, Leonard SW, Schock B, Gohil K, Krueger SK, Cross CE, Williams DE. Alpha-tocopherol modulates Cyp3a expression, increases gamma-CEHC production, and limits tissue gamma-tocopherol accumulation in mice fed high gamma-tocopherol diets. Free Radic Biol Med 38: 773–785, 2005 [DOI] [PubMed] [Google Scholar]
- 70.Troisi RJ, Willett WC, Weiss ST, Trichopoulos D, Rosner B, Speizer FE. A prospective study of diet and adult-onset asthma. Am J Respir Crit Care Med 151: 1401–1408, 1995 [DOI] [PubMed] [Google Scholar]
- 71.Umegaki K, Uramoto H, Esashi T. Lack of influence of a long-term high or low vitamin E diet on the oxidative DNA damage in the bone marrow of mice. Int J Vitamin Nutr Res 67: 149–154, 1997 [PubMed] [Google Scholar]
- 72.van Rijt LS, Lambrecht BN. Dendritic cells in asthma: a function beyond sensitization. Clin Exp Allergy 35: 1125–1134, 2005 [DOI] [PubMed] [Google Scholar]
- 73.van Schayck CP, Smit HA. The prevalence of asthma in children: a reversing trend. Eur Respir J 26: 647–650, 2005 [DOI] [PubMed] [Google Scholar]
- 74.Vander Lugt B, Khan AA, Hackney JA, Agrawal S, Lesch J, Zhou M, Lee WP, Park S, Xu M, DeVoss J, Spooner CJ, Chalouni C, Delamarre L, Mellman I, Singh H. Transcriptional programming of dendritic cells for enhanced MHC class II antigen presentation. Nat Immunol 15: 161–167, 2014 [DOI] [PubMed] [Google Scholar]
- 75.Vanhees K, Coort S, Ruijters EJ, Godschalk RW, van Schooten FJ, Barjesteh van Waalwijk van Doorn-Khosrovani S. Epigenetics: prenatal exposure to genistein leaves a permanent signature on the hematopoietic lineage. FASEB J 25: 797–807, 2011 [DOI] [PubMed] [Google Scholar]
- 76.Vollmer WM, Osborne ML, Buist AS. 20-year trends in the prevalence of asthma and chronic airflow obstruction in an HMO. Am J Respir Crit Care Med 157: 1079–1084, 1998 [DOI] [PubMed] [Google Scholar]
- 77.von Bubnoff D, Fimmers R, Bogdanow M, Matz H, Koch S, Bieber T. Asymptomatic atopy is associated with increased indoleamine 2,3-dioxygenase activity and interleukin-10 production during seasonal allergen exposure. Clin Exp Allergy 34: 1056–1063, 2004 [DOI] [PubMed] [Google Scholar]
- 78.Wang X, Pesakhov S, Harrison JS, Danilenko M, Studzinski GP. ERK5 pathway regulates transcription factors important for monocytic differentiation of human myeloid leukemia cells. J Cell Physiol 229: 856–867, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weiss ST. Diet as a risk factor for asthma. Ciba Fndn Symp 206: 244–257, 1997 [PubMed] [Google Scholar]
- 80.Williams JW, Tjota MY, Clay BS, Vander Lugt B, Bandukwala HS, Hrusch CL, Decker DC, Blaine KM, Fixsen BR, Singh H, Sciammas R, Sperling AI. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat Commun 4: 2990, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wolf G. How an increased intake of alpha-tocopherol can suppress the bioavailability of gamma-tocopherol. Nutr Rev 64: 295–299, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Woollett LA. Maternal cholesterol in fetal development: transport of cholesterol from the maternal to the fetal circulation. Am J Clin Nutr 82: 1155–1161, 2005 [DOI] [PubMed] [Google Scholar]
- 83.Xu H, Oriss TB, Fei M, Henry AC, Melgert BN, Chen L, Mellor AL, Munn DH, Irvin CG, Ray P, Ray A. Indoleamine 2,3-dioxygenase in lung dendritic cells promotes Th2 responses and allergic inflammation. Proc Natl Acad Sci USA 105: 6690–6695, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu H, Zhang GX, Ciric B, Rostami A. IDO: a double-edged sword for T(H)1/T(H)2 regulation. Immunol Lett 121: 1–6, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamaguchi Y, Harker JA, Wang B, Openshaw PJ, Tregoning JS, Culley FJ. Preexposure to CpG protects against the delayed effects of neonatal respiratory syncytial virus infection. J Virol 86: 10456–10461, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yokota A, Takeuchi H, Maeda N, Ohoka Y, Kato C, Song SY, Iwata M. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. Int Immunol 21: 361–377, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]