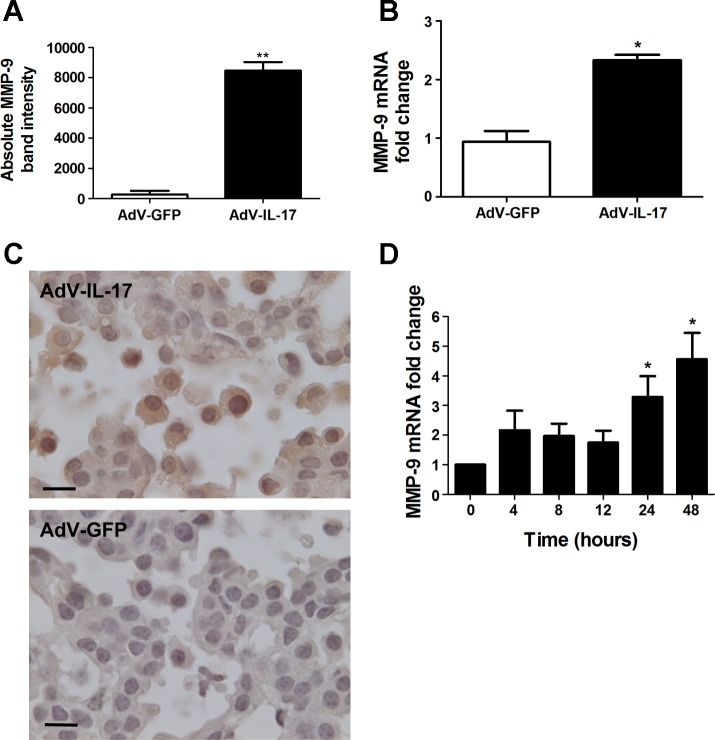
Fig. 3.
Induction of MMP-9 by IL-17. A: wild-type mice were treated with 1×108 pfu AdV-IL-17 or AdV-GFP by oropharyngeal aspiration. One week after treatment, the mice were euthanized and evaluated for MMP-9 expression. Equal volumes of BAL fluid from AdV-IL-17- or AdV-GFP-treated mice were assessed by gelatin zymography for MMP activity (n = 4 per group). Bands corresponding to MMP-9 on the zymogram were quantified by densitometry. Graph shows the MMP-9 band intensity from the BAL fluid of AdV-GFP- (open bar) or AdV-IL-17-treated (solid bar) mice. Data shown are means ± SE. **P < 0.01 AdV-IL-17 vs. AdV-GFP. B: total lung RNA prepared from the mice in A was assessed by qRT-PCR for MMP-9 mRNA levels with β-actin mRNA as the internal control. Mice treated with AdV-IL-17 (solid bar) showed a 2.3-fold increase (2−ΔΔCT method) in MMP-9 mRNA levels compared with AdV-GFP treated littermates (open bar). Data shown are means ± SE; n = 3 per group. *P < 0.05 AdV-IL-17 vs. AdV-GFP. C: K-RasLA1 mice were treated with AdV-IL-17 as described above. One week postexposure the lungs of the treated mice were fixed and paraffin embedded. Lung tissue sections were immunostained with an antibody to MMP-9 by the diaminobenzidine method. MMP-9-positive (brown) staining tumor and immune cells did not appear in the tumor area on an adjacent section stained with the negative control antibody. D: total RNA was prepared from mK-Ras-LE cells at increasing times after treatment with 10 ng/ml IL-17. Graph shows mean levels of MMP-9 mRNA relative to β-actin (2−ΔΔCT method) at the indicated time after addition of IL-17 to the serum-starved cells. The bars represent means ± SE (n ≥ 4). *P < 0.05 relative to the 0 time point.