Figure 3.
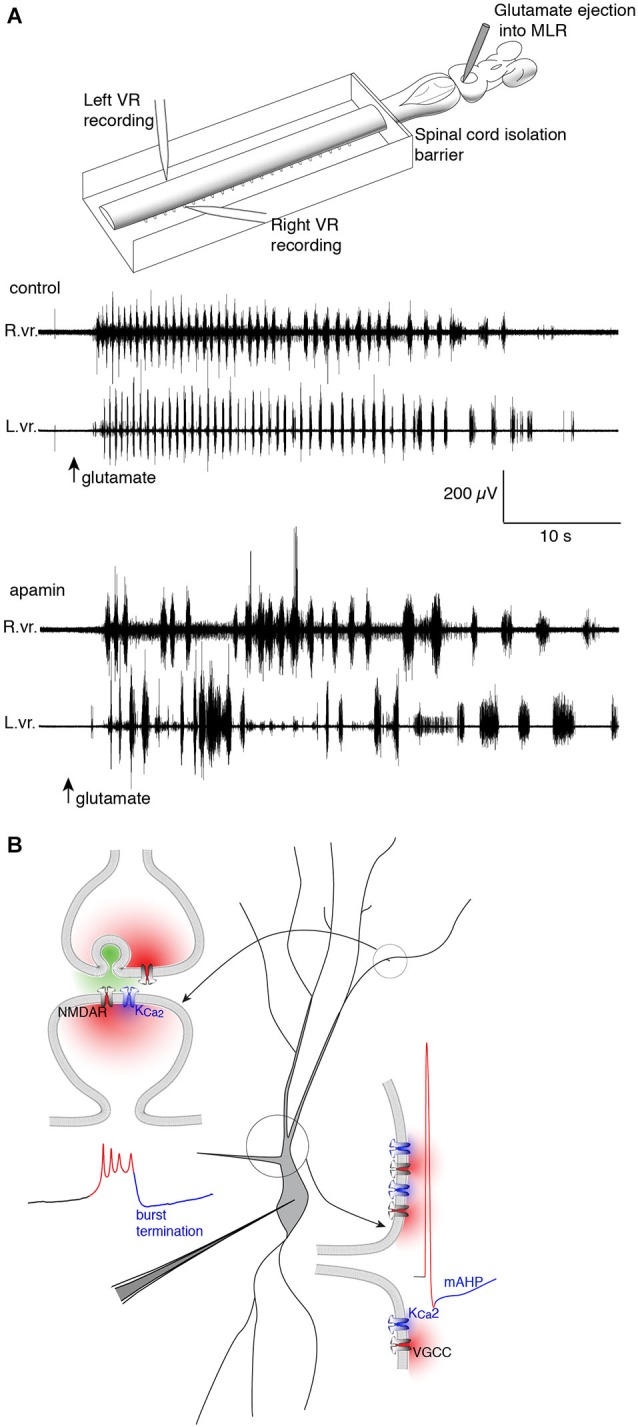
Repolarizing KCa2 channels are spatially segregated in lamprey spinal VHNs according to their function and mechanism of activation. (A) Top: An isolated lamprey CNS can be used to study the brain and spinal circuits controlling locomotion. Pressure-ejection of L-glutamate into the lamprey mesencephalic locomotor region (MLR) induces short episodes of fictive locomotion, the electrophysiological correlate of locomotion. Using a dual-pool recording chamber, pharmacological agents can be selectively applied to the spinal cord, without interfering with descending commands originating in the brainstem that initiate and maintain locomotion. Locomotor bursts are recorded directly from left and right VRs. Bottom: A long locomotor episode with regular, alternating bursts (control) follows after a puff of glutamate into the MLR (arrow, glutamate). Blockade of KCa2 channels with the selective antagonist, apamin, decreases the burst frequency and disrupts the alternating locomotor rhythm. This demonstrates the necessity of KCa2 channels for correct alternation and regularity of the locomotor rhythm (Nanou et al., 2013). (B) The effect of KCa2 channel blockade on locomotion can be explained by the role the channel plays at the cellular level. Within VHNs, KCa2 currents may be evoked either at synapses (top left) whereby synaptic release of glutamate activates NMDAR-mediated Ca2+ entry and thereby closely located KCa2 channels. It is this KCa2-mediated current that is critical for the termination of NMDA-TTX oscillations (blue portion of trace) shown below recorded from somatic microelectrode recordings. KCa2-mediated currents are also responsible for the mAHP seen following action potential firing shown at bottom left. However, this current is activated following Ca2+ entry from VGCCs.
