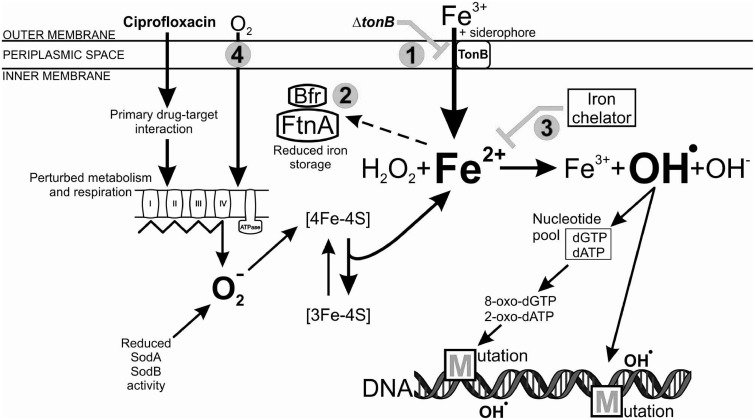
Fig. 6.
Schematic model of iron-overload-dependent oxidative mutagenesis in the Δfur mutant, exposed to a toxic dose of ciprofloxacin. The primary antibiotic-target (ciprofloxacin-DNA gyrase) interaction induces physiological changes in the bacterial cell, including perturbations in metabolism and respiration. The electron transport chain becomes hyperactivated, which stimulates superoxide (O2−) formation. In the absence of Fur, the reduced activity of superoxide dismutases (SodA and SodB) further increases the intracellular level of superoxide. Superoxide damages Fe–S clusters of the proteins leading to the release of Fenton reactive ferrous iron (Fe2+). The constitutive siderophore-mediated ferric iron (Fe3+) uptake and decreased iron storage activity, together with the antibiotic-mediated iron release leads to an intracellular iron overload. Oxidation of the ferrous iron in the Fenton reaction results in the formation of the highly reactive hydroxyl radical (OH.), which may damage nucleotides and DNA, leading to the formation of mutations. Numbers represent intervening points by which antibiotic resistance development could be significantly reduced: 1) Inhibition of siderophore-mediated iron uptake by inactivation of tonB, 2) enhancement of iron storage by overexpressing FtnA and Bfr iron storage proteins, 3) chelation of intracellular free iron using a cell permeable iron chelator o-phenantroline, and 4) applying anaerobic conditions.