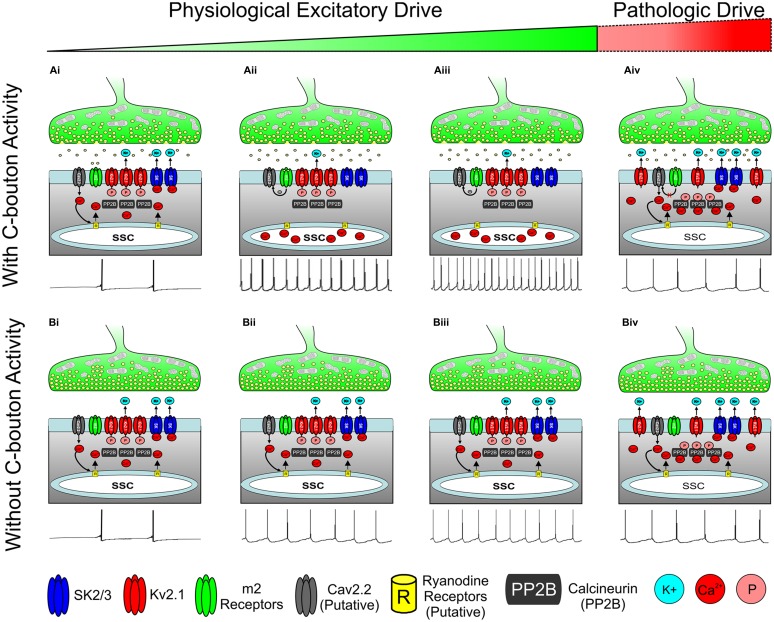
FIGURE 6.
Hypothesis for state dependent regulation of motoneuron activity through the C-Bouton signaling ensemble. (A) C-boutons increase motoneuron firing frequency along a widow of the α-MN activity spectrum. (Ai) With low or transient physiological drive, m2 activation is not likely to mediate an effect on AHP duration or firing rate. (Aii,iii) As excitatory drive increases, persistent m2 receptor activation inhibits local CaV channels through a Gi/Go coupled pathway, preventing both the SK channel activation and Kv2.1 dephosphorylation. Thus, outward K+ current is reduced and neuronal firing rate is increased (relative to Bii and Biii) as illustrated with spike train below. (Aiv) m2-mediated effects on CaV channels are negated by prolonged or repeated membrane depolarization (Hille, 1994) as may occur during extremely strong or pathologic excitatory drive. Here, Ca2+ influx through N-type calcium channels activates SK channels to generate AHP and to dephosphorylate Kv2.1 to increase outward K+ current and reduce firing frequency, as illustrated with spike train below. (Bi–iii) As excitatory drive increases without C-bouton activity, the N-type Ca2+ influx activates SK channels to generate AHP. Thus, the outward K+ current maintains a lower firing frequency than in corresponding images in A. Spike trains illustrated below. (Biv) As in (Aiv), during prolonged or pathologic excitatory drive, N-type Ca2+ influx results in both SK channel activation and Kv2.1 dephosphorylation, thereby increasing outward K+ current and homeostatically decreasing firing rate, illustrated with spike train below. All spike trains depicted in this figure are added for illustrative purposes only and do not represent electrophysiological recordings or computer simulations.