Figure 2.
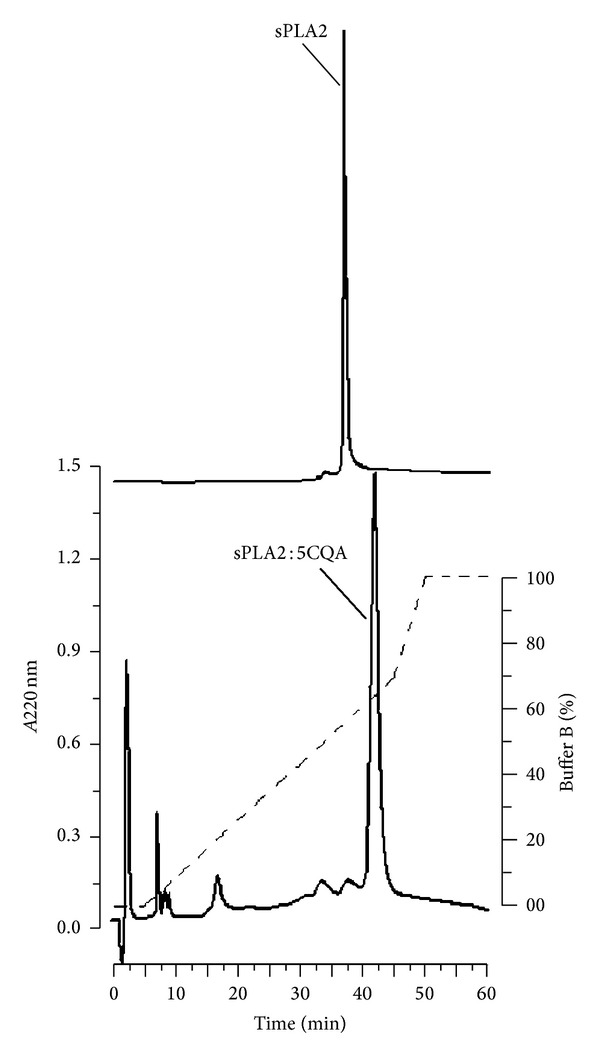
Purification and chemical modification of secretory phospholipase A2 (sPLA2). Fractionation of whole venom was performed by reverse-phase HPLC (C5 column 0.10 cm × 25 cm) using a nonlinear concentration gradient of buffer to obtain a high purity protein. This protein was designated as native sPLA2, which eluted at was eluted with a retention time of 35.8 min. sPLA2 chemically treated with 5-caffeoylquinic acid (5CQA) was subjected to HPLC purification for purification of sPLA2 : 5CQA complex, which it was was eluted at 40.3 min under the same chromatography conditions.
