Fig. 1.
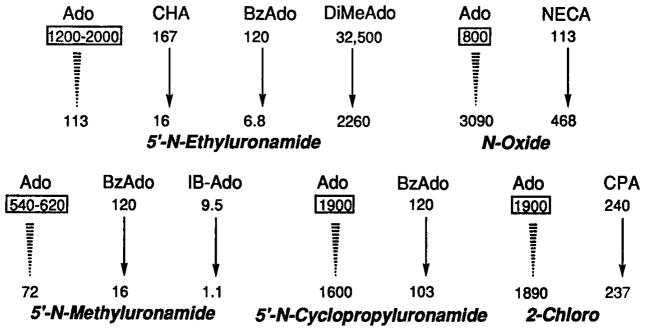
Estimation of the affinity of adenosine at rat A3 receptors using an approach based on comparing Ki values for various adenosine analogs in radioligand binding assays. Estimation of affinity of adenosine at rat A3 receptors was done by extrapolation from ratios of measured affinities of mono- and di-substituted analogs. The estimated value or range for the Ki of adenosine in nM is shown in a rectangular box for each group of compounds. Ki values (26,36, 38) in nM are shown for CHA (N6-cyclohexyl), CPA (N6-cyclopentyl), BzAdo (N6-benzyl), DiMeAdo (N6-dimethyl), IB-Ado (N6-3-iodobenzyl), and NECA (5′-N-ethyluronamide) with or without the modification shown in italics. For example, the transformation of IB-Ado to the corresponding N-methyluronamide, i.e., IB-MECA (second row of arrows, third entry, see Table I for structure), changes the Ki value from 9.5 to 1.1 nM. For the representative compounds selected, 5′-N-Et and 5′-N-Me uronamide modifications resulted in enhancement of affinity by 10- to 18-fold or 8-fold, respectively. 5′-N-Cyclopropyl uronamide and 2-chloro modifications did not markedly change affinities, and the N 1-oxide modification diminished affinity by 4-fold. Thus, the estimated affinity of adenosine is ~ 1 μM.
