Table 2.
Reaction Scope with Respect to Arylamides [a]
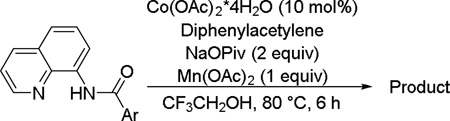 | |||
|---|---|---|---|
| Entry | Ar | Product | Yield, % |
| 1 | C6H5 | 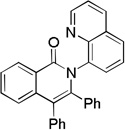 |
78 |
| 2 | 4-CF3C6H4 | 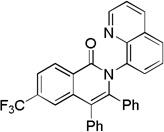 |
70 |
| 3 | 4-BrCC6H4 | 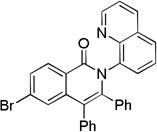 |
73 |
| 4 | 4-NO2C6H4 | 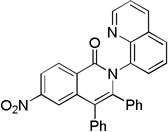 |
78 |
| 5 | 2-MeC6H4 | 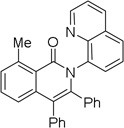 |
86 |
| 6 | 3-IC6H4 | 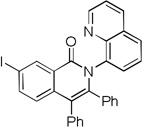 |
84 |
| 7[b] | 2-MeOC6H4 | 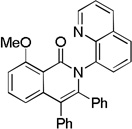 |
74 |
| 8[c] | 2-furyl | 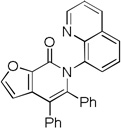 |
81 |
| 9[d] | 2-thiophenyl | 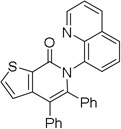 |
86 |
Amide 0.5 mmol, CF3CH2OH 5 mL, air. Yields are isolated yields. Please see Supporting information for details.
Time: 18 h.
Time: 16 h.
Time: 20 h.
