Figure 4. Rhi “tethering” leads to spreading through the target transcription unit, but does not reduce Pol-II occupancy.
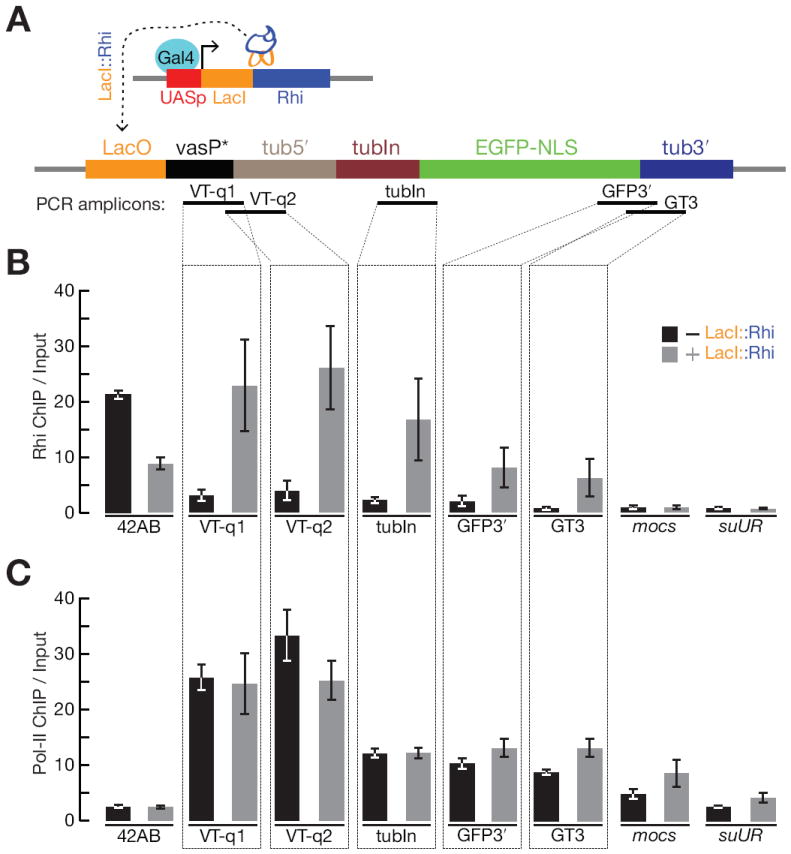
(A) Schematic diagram of the experimental design. An inducible UASp promoter was used to drive expression of the DNA binding protein LacI fused to full length Rhi, in the presence of a reporter gene containing LacI binding sites (LacO) upstream of a promoter (truncated vasa promoter) that drives expression of the 84B alpha tubulin 5′UTR and first intron fused to EGFP with nuclear localization signal (NLS). PCR amplicons indicates positions assayed for Rhi and Pol-II binding in panel B and C.
(B) Fold enrichment by Rhi ChIP across the reporter in the absence (grey) or presence of LacI∷Rhi. The LacI∷Rhi lead to Rhi binding through the transcription unit.
(C) Fold enrichment by RNA pol-II ChIP across the reporter, bars as indicated for panel B. RNA polymerase binding across the transcription unit is not altered in the presence of the LacI∷Rhi. The 42AB locus is used as a positive control for Rhi binding. The mocs and suUR loci are located downstream of reporter construct in the genome. We do not detect Rhi spreading or changes in RNA Pol-II at these loci.
Data are mean ± standard deviation for 3 independent biological samples.
