Fig 1.
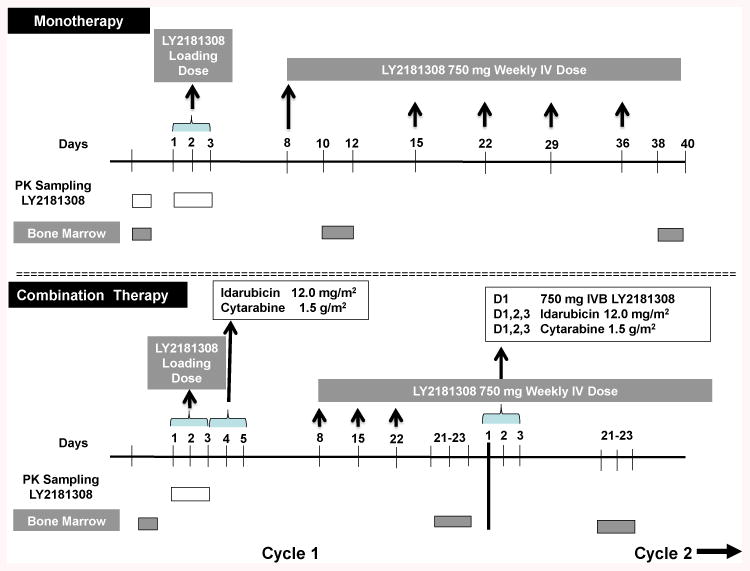
Study Design. Pre amendment- 750 milligrams intravenous bolus dose LY2181308 was administered in loading doses on days 1-3 and day 8. Weekly doses of 750 mg LY2181308 were administered intravenously on days 15, 22, 29, and day 36 thereafter. Post-amendment- 750 milligrams intravenous bolus dose LY2181308 was administered in loading doses on days 1-3 followed by idarubicin 12.0 mg/m2 plus cytarabine 1.5 mg/m2. Weekly doses of 750 mg LY2181308 were administered iv on days 8, 15, and 22 thereafter. Pharmacokinetic and bone marrow sampling are depicted as white and grey boxes, respectively. See methods for details.
