Abstract
Environmental exposure to mercury is suggested to contribute to human immune dysfunction. To shed light on the mechanism we identified changes in the phosphoproteomic profile of the WEHI-231 B cell line after intoxication with Hg2+. These changes were compared to changes in the phosphoproteome that were induced by pervanadate or okadaic acid exposure. Both 250 μM HgCl2 and pervanadate, a known phosphotyrosine phosphatase inhibitor, caused an increase in the number of proteins identified after TiO2 affinity selection and LC-MS/MS analysis. Pervanadate treatment had a larger effect than Hg2+ on the number of Scansite motifs which were tyrosine-phosphorylated, 17, and Ingenuity canonical signaling pathways activated, 4 with score > 5.0. However, Hg2+ had a more focused effect, primarily causing tyrosine-phosphorylation in SH2 domains in proteins that are in the B cell receptor signaling pathway. The finding that many of the changes induced by Hg2+ overlap with those of pervanadate, indicates that at high concentrations Hg2+ inhibits protein tyrosine phosphatases.
Keywords: mercury, Hg2+, toxicology, B cell, WEHI-231, phosphoproteomics, phosphoprotein, phosphotyrosine, TiO2, mass spectrometry
INTRODUCTION
Mercury is distributed in the global environment by both natural geologic processes and human activities 12. Consequently large segments of the world’s population are exposed to the metal through water, air, food, and dental amalgam. Mercury is clearly toxic, and exposure to high or even moderate levels of organic methyl mercury (MeHg), inorganic mercury (Hg2+) or metallic mercury (Hg0) is well established to damage the nervous and immune systems, resulting in neurological and immunological deficiencies in humans 3-5. Epidemiological studies now suggest that exposure to environmental mercury at current common levels, which have been perceived to be non-toxic, may contribute to immune system dysfunction and development or progression of autoimmune disease in humans 6-12. This view is supported by experiments in mouse models of autoimmune disease. In most genetically autoimmune prone mouse strains, exposure to low levels of Hg2+ exacerbates disease 13-16, and in mice not prone to autoimmunity low levels of Hg2+ exacerbate disease in several models of induced (acquired) autoimmunity 6,17,18.
Autoimmune disease arises when autoreactive B and T cells, which are normally held in check by mechanisms collectively known as central and peripheral tolerance, become de-suppressed. Central tolerance refers to the mechanisms by which newly developing immature T cells and B cells are rendered non-reactive to self. These mechanisms are distinct from peripheral tolerance in that they involve the T Cell Receptor (TCR) and B Cell Receptor (BCR) clonotypic receptors and occur only in immature cells, prior to export into the periphery. Peripheral tolerance requires activation of other receptor systems and develops only after T and B cells mature and enter the periphery. Disruption of either mechanism can result in autoimmune disease. Unfortunately most of the studies connecting mercury to autoimmune disease give little guidance as to what the mechanism or mechanisms responsible for the linkage might be. In other words, we do not know how exposure to mercury compromises central and/or peripheral tolerance. This lack of information represents a major gap in our understanding of how environmental mercury interacts with the immune system to promote the development of autoimmune disease.
Recently there has been increased recognition of the essential role played by auto-reactive B cells and of autoantibodies produced by B cell derived plasma cells in the pathology of a variety of autoimmune diseases 19. To a large extent B cell (as well as T cell) tolerance depends upon phosphoprotein mediated signaling. Since mercury inhibits cellular phosphatase activity 20, one possible mechanism for mercury disruption of central tolerance in B cells is the inhibition of critical phosphatases resulting in an aberrant increase in protein phosphorylation. In the studies described here, we have utilized modern mass spectroscopic technology to investigate the effect of mercury on the phosphoproteome in the WEHI-231 mouse B cell line, a well-studied model system known to possess many of the characteristics of immature B cells. The objectives of this study were to determine whether mercury caused an increase in protein phosphorylation in B cells and to identify specific phosphoproteins and their associated signaling pathways most likely to be molecular targets of mercury.
Global phosphoproteomic analysis has recently become possible through the application of affinity selection of phosphopeptides and mass spectrometric identification of those peptides 21-23. Specifically, the WEHI-231 cell line has been profiled resulting in identification of 107 phosphoproteins and 193 phosphorylation sites in one study 24 and over 400 proteins in another 25. This is a relatively modest slice of the entire phosphoproteome as it is predicted that up to 100,000 sites could be phosphorylated in the complete proteome 26. Of equal or even greater importance in the profiling of phosphopeptides is the quantification of the change in phosphorylation after an experimental intervention or environmental exposure. Mass spectrometry enables multiplex analysis by using isotopically distinct labels for different samples 27,28 but can also be used in a label independent way by quantitating based on the number of spectra observed 29,30. For instance a recent study utilizing these methods examining global protein phosphorylation dynamics during deoxyvynivalenol-induced ribotoxic stress in macrophages has identified extensive and unexpected changes to the macrophage phosphoproteome 31. For the work presented in this paper we quantified the abundance of phosphopeptides and phosphoproteins using the spectral counting strategy.
Material and Methods
Materials
Acetic Acid was from BDH Chemicals (through VWR, Radnor, PA), CaCl2 was from Orion, TFA (trifluoroacetic acid) was from Fisher Scientific (Pittsburgh, PA) and formic acid was from EMD. (Billerica, MA). TPCK (L-1-tosylamido-2-phenylethyl chloromethyl ketone)-treated bovine pancreas trypsin and all other reagents including solvents used for HPLC were the highest grade available from Sigma-Aldrich. (St Louis, MO). TiO2, 5 micron, was from GL Sciences.
Cell Culture
WEHI-231 cells were obtained from the American Type Culture Collection. Cells were maintained on RPMI 1640 supplemented with 10% fetal bovine serum, 2 mm glutamine, 50 μM mercaptoethanol, 100 U/ml penicillin and 100 μg/ml streptomycin in a humidified 5% CO2 atmosphere. Cells were passaged three times a week and harvested for experiments while in logarithmic growth.
Exposure and Harvesting
Cells were pelleted then resuspended at 5 × 106 cells per ml for exposure to HgCl2 (Hg2+) at 100 μM or 250 μM, pervanadate at 25 μM or okadaic acid at 100 nM. Following a 10 minute exposure in RPMI 1640 to the test agent the cells were washed twice in ice cold Hanks’ solution then pelleted and frozen. The treatments were divided into two separate experiments: the first experiment included 250 μM Hg2+, 100 nM okadaic acid and an untreated control, the second experiment included 100 μM Hg2+, 25 μM pervanadate and an untreated control. The first experiment was carried out using 3 independent samples per treatment and the second experiment with 4 independent samples per treatment.
Sample Preparation
Cell pellets were lysed in Tris, 20 mM pH 8.0, SDS, 0.5%, LiFl, 1.0 mM and Na3VO4, 0.1 mM then supernatant from a 20,000 × g centrifugation was taken for analysis. Protein in lysates was determined using a BCA assay (bicinchoninic acid assay; Pierce, Rockford, IL). Samples were digested overnight with 1:100 trypsin at a sample protein concentration of 1.0 mg/ml in buffer containing Tris, 20 mM, SDS, 0.1%, LiF, 1.0 mM, Na3VO4, 0.1 mM and 10% acetonitrile. All samples were evaluated by SDS-PAGE to ensure full digestion of the samples before proceeding to phosphopeptide isolation. Phosphopeptides were isolated from 1 mg of digested cell protein using 2 sequential incubations each with 2 mg of 5 μ TiO2 beads for the first experiment.
Phosphopeptide enrichment for the first and second affinity selections was 89 and 93 %, respectively, indicating that additional phosphopeptides could be harvested by increasing the ratio of TiO2 beads to protein without compromising the selectivity. Therefore, the second experiment used a single incubation with 6 mg of 5 μ TiO2 beads for each sample. The selectivity of the 6 mg of 5 μ TiO2 procedure was 98%. Cell digests were incubated with TiO2 in 2% TFA saturated with glutamic acid in 60% acetonitrile. The beads were washed three times with 1% TFA in 60% acetonitrile before eluting phosphopeptides with NH4OH in 50% acetonitrile. TiO2 eluates were neutralized with formic acid, dried under vacuum and stored at −80°C until analysis. Eluted peptides solubilized in 0.1% formic acid were then analyzed by LC-MS/MS without further purification.
Mass Spectrometry
All analyses were performed on a Thermo LTQ equipped with ETD (ThermoFisher Scientific, Watham, MA). Samples were loaded on a peptide Captrap (Michrom, Auburn, CA) trapping column and peptide separations achieved using a linear gradient of 5% to 35% acetonitrile to elute from a Majic 0.1 mm × 150 mm AQ C18 column (Michrom). The first experiment had data from two TiO2 selection steps that were run separately on the mass spec. The .raw files were combined during database searching and a total of 233,195 spectra from 9 samples were submitted for that experiment. The second experiment, with 100 μM Hg2+ or pervanadate, has only one TiO2 elution. Those 12 samples yielded a total of 239,443 spectra. LC-MS/MS for the second treatment group was run in a neutral loss mode so that high abundance precursor neutral losses of 24.25, 32.66, or 49.00 m/z found in an MS2 spectrum were selected for MS3 analysis. The first experiment LC-MS/MS did not incorporate a neutral loss scan.
Database Searching
Tandem mass spectra were extracted by Proteome Discoverer (ThermoFisher Scientific) version 1.4.0.288. Charge state deconvolution and deisotoping were not performed. All MS/MS data were analyzed using Mascot (Matrix Science, London, UK; version 2.4.0) and X! Tandem (The GPM, thegpm.org; version CYCLONE (2010.12.01.1)). Mascot and X!Tandem were each set up to search the 2013_04 release of the SwissProt database (selected for Mus musculus, 16611 entries) assuming the digestion enzyme trypsin. Spectra were searched with a fragment ion mass tolerance of 0.70 Da and a parent ion tolerance of 3.5 Da. The iodoacetamide derivative of cysteine was specified as a fixed modification. Oxidation of methionine, acetylation of the n-terminus and phosphorylation of serine, threonine and tyrosine were specified as variable modifications.
Criteria for Protein Identification
Scaffold (version 4.0.5, Proteome Software Inc., Portland, OR) was used to validate MS/MS based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95.0% probability by the Peptide Prophet algorithm32. Protein identifications were accepted if they contained at least one identified phosphopeptide and had a Protein Prophet probability greater than 80%. Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony. All subsequent analysis of protein sets included all proteins or peptides that met the criteria for identification without weighting for the level of confidence in the identification.
Ascore Filtering
The Ascore algorithm was applied to phosphopeptides that met identification criteria and had multiple possible phosphorylation sites in order to determine which site or sites were likely to be modified and the confidence in those localizations33. Site localization was accepted if the Ascore algorithm indicated at least 95% confidence in the assignment. Peptide identification data were imported into Scaffold PTM (Version 2.1.1, Proteome Software) software where the Ascore algorithm was implemented.
Motif Analysis
Ascore-filtered phosphorylation sites were searched against a database of putative protein kinase target motifs and phosphorylation-dependent binding motifs predicted by Scansite 34. Scansite uses data from oriented peptide library and phage-display experiments to generate position-specific scoring matrices that are queried against sequence databases to predict kinase target and domain binding sites. Our data were searched against the high stringency Scansite data that only include sites that fell within the top 0.2% of scores for each motif. All identified proteins were submitted to the Scansite web service (http://scansite3.mit.edu). Motifs identified in those proteins were retrieved and the invariant residue (the modified residue) for each was searched against our Ascore-filtered phosphorylation sites
Pathway Enrichment Analysis
Sets of proteins that were modified by the treatments were examined for overrepresented pathways using the IPA (Ingenuity Systems Inc., Redwood City, CA) Global Canonical Pathway Analysis (Content version: 14855783 (Release Date: 2013-02-05)). Pathways in the ingenuity canonical pathway database were tested for significant associations with the protein sets described in the results section using Fisher’s exact test with all mouse genes in the ingenuity knowledgebase as background.
Statistical Analysis
Statistical tests were carried out in R version 3.0.0 except for the ANOVA tests of protein normalized spectral counts which were carried out in DanteR version 0.2 35. The sets of modified proteins were maximized for subsequent analysis by accepting statistical test results without correction for multiple testing unless otherwise stated. Proportional Venn diagrams were made with the EulerAPE tool available at http://www.eulerdiagrams.org/eulerAPE/. Where a value and range are reported, those values represent the mean and standard deviation.
RESULTS
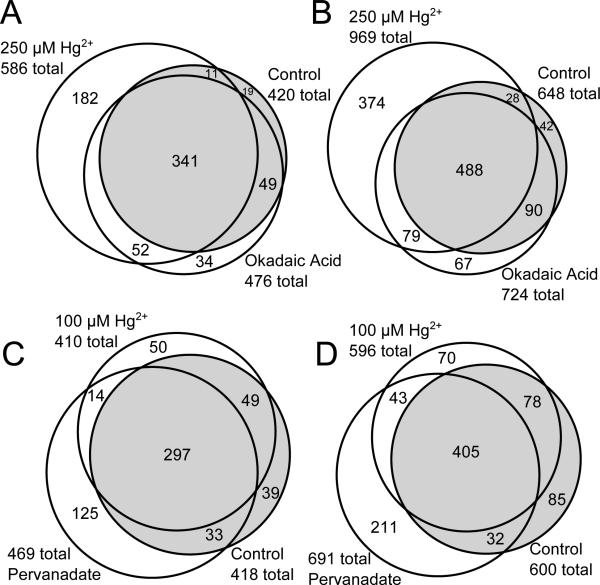
The depth of phosphoproteome coverage and the magnitude of global phosphorylation changes observed in WEHI-231 cells treated with Hg2+, okadaic acid or pervanadate are shown in figure 1. Two separate experiments were run to evaluate the ability of Hg2+ to modulate the phosphoproteome in WEHI-231 cells. The TiO2 selection was effective for phosphopeptides, as indicated by 92% and 98% of all identified peptides being phosphopeptides in the first and second experiment, respectively. In the first experiment, (Figure 1, panels A & B; Supplemental Tables S1 and S3) WEHI-231 cells were exposed to 250 μM Hg2+ or 100 nM okadaic acid for 10 minutes. The concentrations of each agent were selected to achieve a maximal effect on Hg2+-responsive pathways and phosphatase Type-1 and Type-2A substrates, respectively. In this experiment 688 proteins were identified with 95% confidence at the peptide level and a 0.7% false discovery rate at the protein level. The names of proteins in the groups depicted in figure 1 A and B are provided in supplementary tables S1 and S2 respectively. In addition, for proteins that were identified by only one peptide, the Peptide Prophet scores and mass deviations are provided in supplementary tables S3 and S4. All protein identifications and mass spectra have been submitted to the PRIDE database.
Figure 1.
Identification of phosphoproteins and phosphopeptides isolated from WEHI-231 cell. Proteins, panels A & C, or phosphopeptides, panels B & D, identified using TiO2-selected phosphopeptides from untreated cells or cells treated with 250 μM Hg2+ or 100 nM okadaic acid (A & B), or 25 μM pervanadate or 100 μM Hg2+ (C & D). Untreated sample regions are highlighted in grey. Numbers indicate total proteins identified in the group (A & C) or the number of unique phosphopeptides identified in the group (B & D). n = 3 (A & B) or 4 (C & D).
In an attempt to collect additional phosphopeptides and achieve greater depth of coverage the second experiment used 6 mg of TiO2 per mg of protein for the phosphopeptide selection, rather than the 4 mg used in the first experiment. Changing the experimental procedure for TiO2 selection of phosphopeptides had no discernible effect on the number of proteins or peptides identified in any run, indicating that a representative set of phosphopeptides was selected each time. The addition of a neutral loss scan in the second experiment resulted in a 4.3 % decrease in the number of phosphosites identified in control samples with no change in the distribution of spectra based on the type of phosphorylated residue. This indicates that for the TiO2 selected samples in this study the depth of coverage was limited by the degree of chromatographic fractionation after phosphopeptide selection.
A review of the literature has shown that mercury can interact with cellular systems in a great variety of ways 36. It is also known that protein phosphatases, and protein tyrosine phosphatases in particular, are susceptible to oxidation because they contain cysteine residues in their active sites 37. As mercury is well known to irreversibly bind to cysteine residues38, we hypothesized that one prominent mechanism by which mercury could modulate the phosphoproteome would be via tyrosine phosphatase inhibition. In addition, several phosphoserine/phosphothreonine phosphatases are sensitive to oxidative stress39. Mercury could act to inhibit phosphatase activity by direct sulfhydryl binding in active site Cys residues or indirectly via oxidative stress.
An increase in cellular protein phosphorylation should result in an increase in the number of phosphoproteins and phosphopeptides detected by mass spectrometry after TiO2 phosphopeptide selection. When the data for Hg2+ exposed cells were compared to control, the 250 μM Hg2+ caused the number of phosphoproteins and unique phosphopeptides to increase by 166 and 321. Normalized spectral counts were used to measure protein abundance and ANOVA to identify proteins that had altered abundance in response to treatment. This analysis identified 180 proteins that had changes (p < 0.05) in spectral count abundance. Treatment with 250 μM Hg2+, in addition to increasing the number of peptides and proteins identified, also increased the variability in the proteins that were identified between replicate runs so that 30.0% of the proteins identified in 250 μM Hg2+ treated cells were observed in just one of the three samples while only 21.4% were identified in just one control sample and 24.0% of proteins were identified in just one of the okadaic acid treated samples. Proteomics experiments using data dependent analysis typically identify variable sets of proteins between technical replicates so that 30% or more of proteins in one analysis won’t be found in a replicate analysis 40. The data presented here were from independent biological samples that are expected to have greater variability than technical replicates so the estimate of 30% variability between samples represents a reasonable best case outcome.
In the second experiment (Figure 1, panels C & D; Supplemental Tables S2 and S4), the Hg2+ concentration was decreased to 100 μM to achieve greater specificity of action and pervanadate, a known inhibitor of phosphotyrosine phosphatases, was introduced as a positive control. Pervanadate acts, as we hypothesize mercury acts, by two mechanisms for inhibition of pTyr phosphatase activity: directly by competitive phosphatase inhibition or indirectly by oxidative inactivation41. It was used at 25 μM to achieve maximal inhibition of phosphotyrosine phosphatases. In the second experiment 607 proteins were identified with 95% confidence at the peptide level and a 1.8% false discovery rate at the protein level. Pervanadate treatment increased the number of phosphoproteins by 51 and phosphopeptides by 91 over control. ANOVA identified 160 proteins that had changes in normalized spectral counts (p < .05) due to pervanadate treatment and 53 that had changes due to 100 μM Hg2+. Like Hg2+, pervanadate increased the variability of protein identifications between samples, resulting in 24.7% of all identified proteins being identified in just one out of four samples, compared to 18.9% in controls. Cells treated with 100 μM Hg2+ had 22.4% of their proteins identified in just one out of four samples.
In the two experiments the 250 μM Hg2+ and the 25 μM pervanadate groups have the greatest number of phosphoproteins and phosphopeptides identified. In contrast, the actions of 100 nM okadaic acid and 100 μM Hg2+ were very modest. Okadaic acid caused an increase of only 56 proteins and 76 peptides over control and the 100 μM Hg2+ exposure group had a decrease of 8 proteins and 4 peptides compared to control (table 1). Protein spectral counts were compared between okadaic acid treated and control cells using ANOVA and 37 proteins were found to be different at the p<0.05 level. Those proteins are highlighted in gray in supplementary table S1. Table 1 shows the number of proteins and the number of peptides that change for each treatment group as well as the number that are significantly different from control or that change by 2 fold or greater in spectral counts.
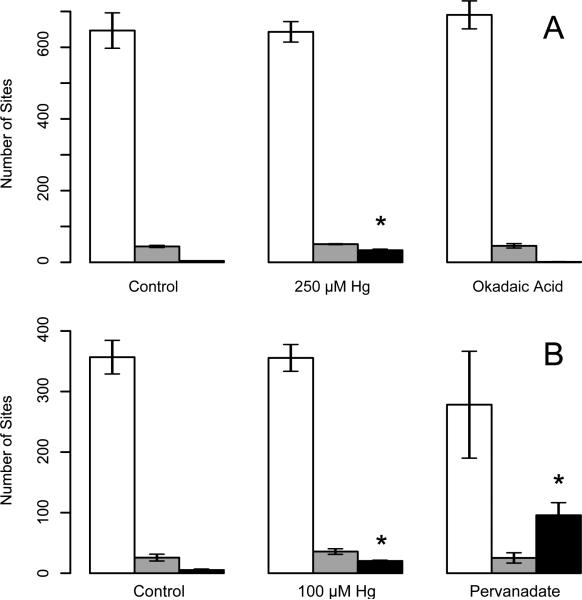
The Ascore algorithm33 was used to localize phosphorylation sites with high-confidence. For the site assignment analysis, peptides were not assembled into proteins and no attempt was made to find the minimal list of proteins required to explain all matched spectra. If a sequence appeared in more than one protein then all possible protein assignments were included in the set of identified proteins for subsequent analysis. In the 250 μM Hg2+/okadaic acid experiment, 2136 possible phosphorylation sites were submitted for Ascore analysis which determined that 1388 of them, 65.0%, had site localization evidence that surpassed 95% confidence. In the 100 μM Hg2+/pervanadate experiment, 891 of 1641 possible phosphorylation sites, 54.3%, had greater than 95% site localization confidence. Data were aggregated by treatment to determine the assignment of pSer, pThr and pTyr residues. Over 90% of phosphopeptide sites identified were pSer in the control samples and the ratio of pSer : pThr phosphopeptides was constant at 12 : 1 for all conditions evaluated. The number of pTyr phosphopeptides identified was increased by the exposure to 100 μM or 250 μM Hg2+ and most dramatically by exposure to 25 μM pervanadate, (figure 2). The number of pTyr peptides identified increased from an average of 5.3 ± 1.7 in controls to 20.3 ± 1.3 and 95.8 ± 20.8 in the 100 μM Hg2+ or pervanadate treatment groups, respectively. In the first experiment the number of pTyr peptides identified increased from 4.0 in control to 33.7 ± 3.1 and 1.3 ± 0.6 in the 250 μM Hg2+ or okadaic acid treatment groups, respectively. It is notable that although the exposures to Hg2+ increased the number of different phosphoproteins and phosphopeptides identified without changing the number of different pSer sites identified using the Ascore algorithm (figure 2), the number of spectral counts assignable to pSer residues in the 250 μM Hg2+group is smaller than in the control group (1916±59 in 250 μM Hg2+vs 2570±64 in controls, t-test p-value=0.0018).
Figure 2.
Hg2+ and pervanadate increase tyrosine phosphorylation for Ascore-filtered sites. Panel “A” shows the number of pSer (white bars), pThr (grey) and pTyr (black) sites identified in each group for the first exposure condition, n = 3. Panel “B” shows similar data for the second exposure condition, n=4 ± Std. Dev, * indicates that pTyr is significantly different from control (t-test, p<0.05).
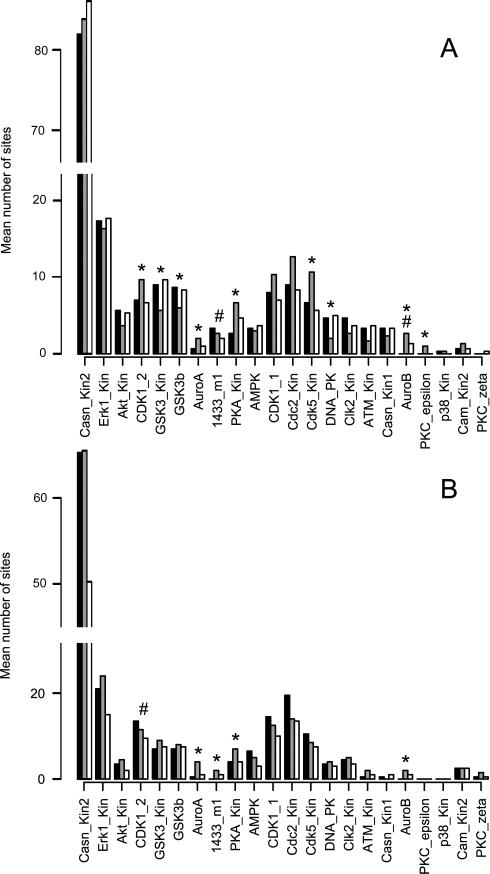
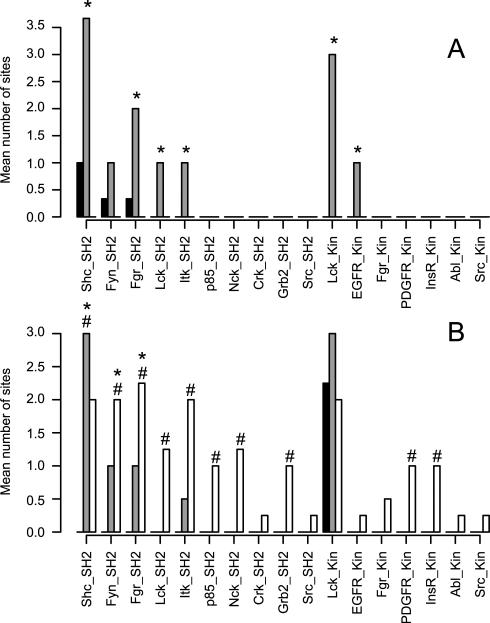
Two approaches were used in evaluating the proteins involved in the cellular response to Hg2+: motif analysis using Scansite 34 and pathway analysis using IPA. For the motif analysis, the Ascore-filtered sites were searched against the Scansite database and then the numbers of sites that matched each Scansite motif were counted. For presentation, the data were split into pSer/pThr sites (figure 3) and pTyr sites (figure 4). In the 250 μM Hg2+/okadaic acid experiment, 332 of 1461 (23%) sites searched had identified motifs (figure 3 A and 4 A). In the 100 μM Hg/pervanadate experiment, 226 of 943 (24%) had motif assignments (figure 3 B and 4 B). Scansite has high specificity and low sensitivity relative to other phosphosite prediction algorithms and assignment of kinase motifs for less than 50% of the submitted sites is reasonable42.
Figure 3.
ScanSite motif analysis of Ser/Thr phosphorrylation sites. Identifed pSer and pThr phosphorrylation sites were searched against the ScanSite database. The pSer/pThr phosphorylation sites that match each motif were summed and the averages are shown abouve. Black bars indicate untreated, grey bars indicate 250 μM Hg2+ and white bars indicate okadaic acid treated cells for panel A, n=3. Black bars indicate unttreated, grey bars indicate 100 μM Hg2+ and white bars indicate pervandate treated cells for panel B, n=4. * indicates that Hg2+ treatment was different from control and # indicates that okadaic acid treatment or pervanadate treatments were different from control using ANOVA followed by Tukey's test, p<0.05.
Figure 4.
Identified Try phosphorylation sites were searched against the ScanSite database. Matching pTyr sites for each motif were summed and the averages are shown. Black bars indicate untreated, grey bars indicate 250 μM Hg2+ and white bars indicate okadaic acid treated cells for panel A, n=3. Black bars indicate untreated, grey bars indicate 100 μM Hg2+ and white bars indicate pervanadate treated cells for panel B, n=4. * indicates that Hg2+ treatment was different from control and # indicates that prevanadate treatment was different from control using the Kruskal-Wallis test (p<0.05).
In the combined data set from all treatments, the motifs for 10 SH2 binding domains and 7 kinases were discovered. The pTyr motifs that were found in cells treated with 250 μM Hg2+ were mainly binding sites for SH2 domain proteins. This is consistent with the predominance of SH2 domains in phosphotyrosine signaling43. Nine sites were in SH2 motifs including Y182 and Y193 of CD79A and Y195 and Y206 of CD79B, which constitute the ITAM domains of those proteins, Y935 of PTPRC (Receptor-type tyrosine-protein phosphatase C) and Y342 of KSYK (Tyrosine-protein kinase SYK). Several of those sites, including Y182 and Y193 of CD79A, Y935 of PTPRC and Y342 of KSYK, were also found among the SH2 motifs in 100 μM Hg2+ treated cells.
Phosphoproteins that had different spectral count abundance as determined by ANOVA, p<0.05, were submitted for canonical pathway analysis in Ingenuity. The top scoring, non-redundant pathways for Hg2+ and pervanadate treatments are shown in table 2. The B cell receptor signaling pathway was the top hit for 100 μM Hg2+ and pervanadate treated cells and the second hit for 250 μM Hg2+ treated cells. The eIF2 signaling pathway was the top hit for 250 μM Hg2+ treated cells. Phosphoproteins with abundance changes induced by okadaic acid treatment had lower scores for pathway enrichment than the other treatments: eIF2 signaling was its second highest hit.
Table 2.
Ingenuity pathway analysis of proteins with altered abundance in response to treatment
| 100 μM Hg2+ |
250 μM Hg2+ |
Okadaic acid |
Pervanadate |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Pathway Name | Proteins Identified | Scorea | # | Score | # | Score | # | Score | # |
| eIF2 Signaling | EIF3G, RPS3A, EIF3B, MAPK1, GRB2, MAPK3, EIF5, RPL22L1, RPLP2, RPLP0, RPLP1, EIF3D | 2.21 | 3 | 3.95 | 8 | 1.78 | 2 | 6.88 | 11 |
| B Cell Receptor Signaling | BLNK, BTK, PTPN6, CD79B, MAPK1, GRB2, MAPK3, PLCG2, SYK, CD22, GSK3A, CD79A | 6.14 | 6 | 3.57 | 7 | - | - | 8.64 | 12 |
| RAN Signaling | KPNA3, RANGAP1, RANBP2 | - | - | 3.51 | 3 | - | - | - | - |
| Systemic Lupus Erythematosus Signaling | PTPN6, CD79B, MAPK1, GRB2, MAPK3, PLCG2, CD72, CD22, HLA-B, HNRNPC, CD79A | 5.27 | 6 | 2.05 | 6 | - | - | 6.05 | 11 |
| Phospholipase C Signaling | BLNK, BTK, CD79B, MAPK1, ARHGEF2, FNBP1, CD79A, PRKCB, SYK, HDAC1, MARCKS, MAPK3 | 5.18 | 6 | 3.28 | 8 | - | - | 5.90 | 11 |
Ingenuity reports a score that is the negative log (p-value)
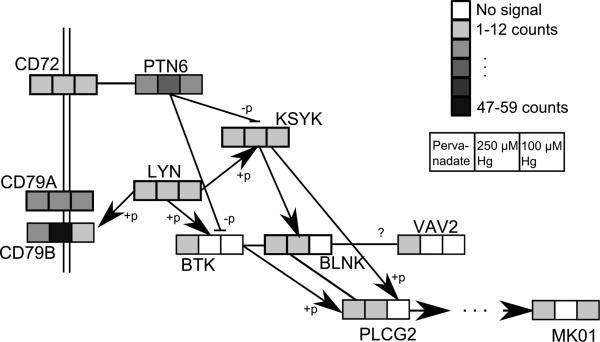
Pathway and cellular process enrichment for proteins that contained phosphotyrosine residues was evaluated by submitting proteins with Ascore-filtered Tyr phosphosites that had at least 3 spectral counts in any replicate to Ingenuity canonical pathway analysis, (table 3). Ten proteins met the submission criteria for 250 μM Hg2+ treated cells, 4 for 100 μM Hg2+ treated cells and 32 for pervanadate treated cells. The four pTyr proteins from the 100 μM Hg2+ group that met the selection criteria are all associated with the B-cell receptor signaling pathway. That pathway is also the top scoring pathway for the 250 μM Hg2+ group where it is separated from the next highest scoring pathway by almost 7 order of magnitude change in p-value. Pervanadate treated cells had tyrosine-phosphorylated proteins in several other pathways including 14-3-3-mediated signaling, remodeling of epithelial adherens junctions and axonal guidance. Figure 5 is an adaption of the KEGG map44 for the B-cell receptor signaling pathway that indicates the level of tyrosine phosphorylation for identified proteins. All of the proteins in the KEGG pathway map that were identified as phosphoproteins are included irrespective of the findings from the Ingenuity analysis.
Table 3.
Ingenuity pathway analysis of pTyr containing proteins
| 100 μM Hg2+ |
250 μM Hg2+ |
Pervanadate |
|||||
|---|---|---|---|---|---|---|---|
| Pathway Name | Proteins Identified | Scorea | # | Score | # | Score | # |
| B Cell Receptor Signaling | BLNK, BTK ,PTPN6, MAPK1, PLCG2, SYK, CD79A, VAV1 | 6.1 | 3 | 10.3 | 6 | 7.87 | 7 |
| 14-3-3-mediated Signaling | TUBA1A, MAPK1, PRKCD, PLCG2, TUBA1C, TUBA1B | - | - | - | - | 7.2 | 6 |
| Remodeling of Epithelial Adherens Junctions | TUBA1A, MAPRE1, TUBA1C, TUBA1B | - | - | - | - | 5.2 | 4 |
| Axonal Guidance Signaling | TUBA1A, MAPK1, CFL1, PRKCD, PLCG2, TUBA1C, TUBA1B | - | - | - | - | 5.0 | 7 |
| Ephrin A Signaling | CFL1, VAV1 | - | - | 3.5 | 2 | - | - |
Ingenuity reports a score that is the negative log (p-value)
Figure 5.
Adaptation of the KEGG B-cell signaling pathway including protection spectral counts. Boxes indicate prevanadate, 250 μM Hg2+ and 100 μM Hg2+ with the color intensity showing the average total spectral counts for that protection. Control samples had 2 or fewer spectral counts for all proteins shown. Proteins are labeled by their Uniprot entry name with the suffix “_MOUSE” removed. +p and –p indicate phosphorylation and dephosphorylation.
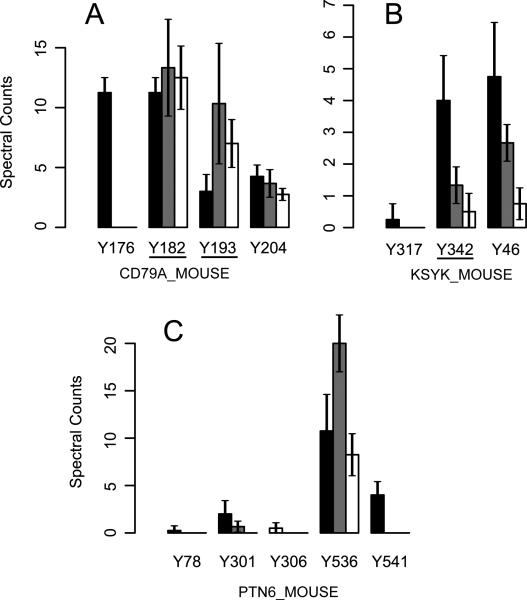
Phosphotyrosine sites identified in the Hg2+ treated cells were concentrated in just a few proteins. Of the average 90 Ascore-filtered spectral counts spread over 38 proteins in the 250 μM Hg2+ treated cells, 54% ± 8.7 of these were in the two protein, PTN6 and CD79A. Similarly, 57% ± 3.9 of pTyr spectral counts in 100 μM Hg2+ treated cells were found in those same two proteins. In pervanadate treated cells, the proteins PTN6 and CD79A accounted for an average of only 12% ± 0.9 of pTyr spectral counts.
Figure 6 shows pTyr spectral counts at sites in selected proteins in the B-cell signaling pathway. Phosphorylated CD79B total spectral counts were greatly increased by Hg2+ treatment (figure 5), and all spectral counts for that protein came from the peptide spanning A187 to R213_which contains its ITAM signaling domain. The Ascore filter removed 98.0% of its possible site-based spectral counts so this protein had a low signal in Ascore filtered data and is not included in this figure.
Figure 6.
Pervanadate and Hg treatment differentially increase pTyr. The number of Ascore filtered spectral counts for tyrosine phosphopeptides are shown, error bars indicate standard deviation. Control samples had no more than one spectral count for any of the sites shown. Black bars indicate pervanadate treated cells (n=4), grey bars 250 μM Hg2+ treated cells (n=3) and white bars represent cells treated with 100 μM Hg2+ (n=4). SH2 domains identified by Scansite are underlined.
Discussion
We used a ten minute exposure to high doses of Hg2+ to explore the hypothesis that it would act as an inhibitor of phosphatases and so increase protein phosphorylation in the WEHI-231 B cell model. Exposure to 250 μM Hg2+ had a profound effect on the phosphoproteome of WEHI-231 cells. The numbers of phosphoproteins and phosphopeptides were increased in comparison to controls (figure 1, table 1) as was the number of pTyr sites identified (figure 2) and the abundance of phosphoprotein components of the B cell receptor signaling pathway were dramatically increased (figures 5 and 6). This pathway is intimately involved in regulating B cell tolerance, the mechanism by which the B cell repertoire is pared of self-reactive B cells45. Not surprisingly, disruption of the B cell receptor pathway has been shown to be linked to autoimmune disease46,47. We have presented evidence that Hg2+ can increase protein phosphorylation in a B cell model, particularly in components of the B cell receptor signaling pathway, suggesting this as a possible mechanism for disruption of central tolerance by mercury.
Neutral loss scans were included in the second experiment in an attempt to increase the depth of coverage for phosphoproteins. For the entire data set for experiment two, when the neutral loss scans are included, 239,443 spectra were submitted resulting in 607 phosphoproteins and 891 phosphosites identified. Without the neutral loss scans 187,716 spectra were submitted and 581 proteins and 853 phosphosites were identified. The modest increase in identified phosphoproteins and phosphosites when neutral loss spectra were included shows that the addition of those spectra were not a major factor in expanding the depth of coverage for the phosphoproteome. The possibility that the additional time required for acquiring neutral loss spectra in the experiment would decrease the depth of coverage of all phosphoproteins or of phosphopeptides with a specific type of residue, such as pTry peptides, can not be completely discounted. This possibility is supported by data for the total number of phosphoproteins and phosphopeptides identified in the untreated samples. In the two experiments 343 ± 13 and 306 ± 16 phosphoproteins and 535 ± 28 and 428 ± 24 phosphopeptides were identified in the experiments without and those with neutral loss scans, respectively. Adding the neutral loss scan did not change the ratio of pSer/pThr to pTry phosphopeptides that were identified, pTyr represented 3.1 ±0.49 % of all phosphorylated residues assigned by the ASCORE algorithm when the neutral loss scan was included and 3.4 ±0.31 % when those scans were not submitted for analysis. The pervanadate and the 100 μM Hg treatment groups were both in the experiment that incorporated neutral loss scans and they have the highest and second lowest number of pTyr sites identified. Taken together these data indicate that including the neutral loss scans in the experiment did not have a major effect on either the depth of phosphoproteome coverage or on the type of phosphosite that was detected. The limiting factor for the depth of coverage for this analysis was, therefore, the degree of fractionation after the phosphopeptide selection step. Since, in all instances, this was a single 2 hour gradient on a reversed phase column, the results from the two experiments are comparable.
Exposure to Hg2+ at the lower concentration of 100 μM caused a slight decrease in the numbers of phosphoproteins and phosphopeptides identified in comparison to untreated cells (table 1). The 100 μM Hg exposure group did have an increase in pTyr sites identified (figure 2) and an increase in the abundance of phosphoproteins and pTyr sites in the B cell signaling pathway (figures 5 and 6, table 3). This confirms that increased pTyr signaling in the B cell receptor pathway is a core effect of Hg2+ in WEHI-231 cells. Although Hg2+ would be expected to bind to most protein cysteine residues after exposure to either the 100 or 250 μM concentrations used for these studies, Hg2+ is positively charged and it has been shown that the ability of Hg2+ to cross the plasma membrane is a function of concentration. Because of this barrier, at lower exposure levels the cytoplasmic Hg2+ availability is more limited 48. It is possible that the effects of Hg2+ on the phosphoproteome which we find after 100 μM exposures are limited to the consequences of Hg2+ binding to external cysteine, while the differences observed after exposure to 250 μM Hg2+ reflect changes due to Hg2+ binding to both the external and the cytoplasmic cysteines.
In order to establish the effects of exposing cells to known phosphatase inhibitors we treated WEHI-231 cells with okadaic acid and pervanadate. Okadaic acid is the prototypical phosphatase inhibitor that has high specificity for the major pSer/pThr phosphatases whereas pervanadate inhibits pTyr phosphatases. The effect of okadaic acid on the WEHI-231 phosphoproteome was modest in comparison to the other treatments used (figure 1) and only the AuroB phosphorylation motif was increased in representation in the data by okadaic acid. Although 100 nM okadaic acid is typically a maximally effective concentration for inhibiting both Type-1 and Type-2A pSer/pThr phosphatases, in our experiment the full effect of that inhibition may not have been observed because the duration of exposure may have been insufficient to allow a general increase in the phosphorylation state of cellular proteins in the unstimulated cell system we used for these studies. Hg2+ and pervanadate are less specific and act on a broader range of substrates as well as subjecting the cells to an oxidative environment that would cause an activation of signaling pathways in the cells as they respond to the oxidative stimulus. Pervanadate, like 250 μM Hg2+ stimulated a robust response in the WEHI-231 cells. The numbers of phosphopeptides, phosphoproteins and pTyr sites were all increased in comparison to untreated cells (figures 1 and 2 and table 1). The abundance of phosphoproteins and the number of pTyr sites in the B cell signaling pathway were both increased (figures 5 and 6, tables 2 and 3). Overall, the phosphoproteomes of 250 μM Hg2+ treated cells and pervanadate treated cells are similar to each other, consistent with the hypothesis that they share the same mechanism of action. This is consistent with Hg2+ inhibiting pTyr phosphatases through inactivation of cysteines in the active sites of pTyr phosphatases as is well established for pervanadate, but the action of Hg on the phosphoproteome may not be limited to this one mechanism However the two treatments differ from each other in two ways. First, 250 μM Hg2+ had some effect on Ser/Thr signaling as indicated by the enrichment of 9 pSer/pThr motifs in 250 μM Hg2+ treated cells compared to untreated while pervanadate treatment had a smaller effect with the enrichment of only 4 pSer/pThr motifs (figure 3). Second, pervanadate stimulated the phosphorylation of a broader range of Tyr residues than did the Hg2+ treatment. This is indicated by the greater number of pTyr residues identified in pervanadate treated cells in comparison to 250 μM Hg2+ treated cells (95.8 vs. 33.7 sites, figure 2) and the greater number of pTyr motifs that were enriched in pervanadate treated cells in comparison to Hg2+ treated cells (10 motifs compared to 6 motifs, figure 4). In addition, as noted above, over 50% of the Ascore filtered pTyr spectral counts in cells treated with either concentration of Hg2+ were accounted for by just two proteins in the B cell receptor signaling pathway, PTN6 and CD79A, while those proteins accounted for only 12% of pTyr spectral counts in pervanadate treated cells. These data suggest that Hg2+ has a more narrow range of targets in pTyr stimulation in comparison to pervanadate.
Up to this point our findings are consistent with the view that pervanadate and Hg2+ each modulate Tyr phosphorylation by inhibiting phosphotyrosine phosphatases. The mechanism for that inhibition is likely to be redox dependent inactivation of cysteines that are present in the active site of the protein tyrosine phosphatases. In fact, earlier work based on anti-pTyr western blots found that exposure of lymphocytes to similar concentrations of Hg2+ as were used here also resulted in extensive redox-linked ligand-independent increases in protein tyrosine phosphorylation 50,51. Pervanadate was the most effective of all the agents tested in increasing both the number of pTyr peptides identified and the number of pTyr motifs. However, both concentrations of Hg2+ tested also increased the number of pTyr peptides and motifs identified.
Phosphotyrosine identification in Jurkat and Primary T-cells was able to identify up to 299 proteins with phosphotyrosine 52. However the majority of studies found between 75 and 168 phosphotyrosine sites 52. In WEHI-231 cells 193 phosphorylation sites were identified and mapped to 107 proteins using IMAC 24. Given the 679 phosphoproteins and the 1168 phosphorylation sites identified in this study it is clear that these data represent a meaningful examination of the phosphoproteome and the actions of pervanadate and mercury upon it.
The massive disruption of the phosphoproteome after exposure of WEHI-231 cells to high levels of Hg2+ is consistent with the observation that, at high levels, Hg2+ is extremely toxic, leading to rapid cell death4. This is consistent with the finding that proteins in the eIF2 signaling pathway were more abundant in the phosphopeptides isolated from cells treated with Hg2+. Phosphorylation of eIF2 prevents that protein from initiating protein synthesis and is an indicator of cell stress49. However, at lower and more environmentally relevant levels, Hg2+ is not cytotoxic but, nevertheless, is linked with adverse autoimmune effects 6-12. A likely explanation for this is that at lower concentrations, Hg2+ acts much more selectively.
Supplementary Material
Table 1a.
Summary of phosphoproteins identified in and their changes in abundance in response to treatment
|
Changed Compared to Control |
|||||
|---|---|---|---|---|---|
| Proteins | Proteins unique to Treatment | ANOVA (p<0.05) | >2 fold spectral count increase | >2 fold spectral count decrease | |
| Control | 420 | 19 | |||
| 250 μM Hg2+ | 586 | 182 | 180 | 48 | 75 |
| Okadaic acid | 476 | 34 | 37 | 9 | 16 |
| Control | 418 | 29 | |||
| 100 μM Hg2+ | 410 | 50 | 53 | 13 | 12 |
| Pervanadate | 469 | 125 | 160 | 42 | 49 |
Table 1b.
Summary of phosphopeptidesa identified and their changes in abundance in response to treatment
|
Changed Compared to Control |
||||||
|---|---|---|---|---|---|---|
| Phospho-Peptides | Peptides unique to treatment | ANOVA (P<0.05) | >2 fold spectral count increase | >2 fold spectral count decrease | Phospho Peptides % | |
| Control | 648 | 42 | 90.8 | |||
| 250 μM Hg2+ | 969 | 374 | 337 | 43 | 70 | 92.5 |
| Okadaic acid | 724 | 67 | 71 | 7 | 9 | 93.2 |
| Control | 600 | 85 | 97.6 | |||
| 100 μM Hg2+ | 596 | 70 | 65 | 7 | 7 | 98.0 |
| pervanadate | 691 | 211 | 212 | 45 | 31 | 98.0 |
All phosphopeptides were assigned to a protein in table 1a
ACKNOWLEDGMENT
This work was performed in the Wayne State University and Karmanos Cancer Center Proteomics Core that is supported by P30 CA022453. The Ingenuity Pathway Analysis license was provided through the Applied Genomics Technology Center (AGTC) at Wayne State University. The AGTC resources are funded, in part, by the NIH Cancer Center Support Grant (NIH P30 CA22453).
Funding Sources
This work was funded through a grant from the NIH to AR: R21 ES019228. All mass spectrometry was done on an instrument funded by a grant to PMS; S10 RR020893.
ABBREVIATIONS
- pTyr
phosphotyrosine
- pSer
phosphoserine
- pThr
phosphothreonine
- RPMI
Roswell Park Memorial Institute
- SDS-PAGE
sodium dodecyl sulphate polyacrylamide gel electrophoresis
- BCA assay
bicinchoninic acid assay
- ETD
electron-transfer dissociation
- LC-MS/MS
liquid chromatography with tandem mass spectrometry
- ANOVA
analysis of variance
- ITAM
(immunoreceptor tyrosine-based activation motif)
- TCR
T cell receptor
- BCR
B cell receptor
- SH2
src homology 2
- KEGG
Kyoto encyclopedia of genes and genomes
- IPA
Ingenuity pathways analysis
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. Nicholas J. Caruthers: data analysis, interpretation of findings and manuscript preparation. Paul M. Stemmer: study design, sample preparation, data analysis, interpretation of findings and manuscript preparation. Namhee Shin: sample preparation and mass spectrometry. Alan Dombkowski: data analysis. Joseph A. Caruso: mass spectrometry and data analysis. Randal Gill: sample preparation. Allen Rosenspire: study design, interpretation of findings and manuscript preparation. All authors have given approval to the final version of the manuscript.
ASSOCIATED CONTENT
All proteins identified along with their protein probabilities and the number of peptides assigned to that protein are listed in supplementary tables S1: ”Proteins Identified in the 250 μM HgCl2/okadaic acid experiment”, and S2: ”Proteins Identified in the 100 μM HgCl2/pervanadate experiment”. For all proteins with only one peptide assignment the information regarding the quality of the identifications is listed in supplementary tables S3: ”Peptide identification data for proteins found in the 250 μM Hg2+/okadaic acid experiment by only one peptide” and S4: ”Peptide identification data for proteins found in the 100 μM HgCl2/pervanadate experiment by only one peptide”. This material is available free of charge via the Internet at http://pubs.acs.org. The mass spectrometry data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository53 with the dataset identifier PXD000312 and DOI 10.6019/PXD000312.
Reference List
- 1.Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D. Environmental exposure to mercury and its toxicopathologic implications for public health. Environ. Toxicol. 2003;18(3):149–175. doi: 10.1002/tox.10116. [DOI] [PubMed] [Google Scholar]
- 2.Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D. Environmental exposure to mercury and its toxicopathologic implications for public health. Environ. Toxicol. 2003;18(3):149–175. doi: 10.1002/tox.10116. [DOI] [PubMed] [Google Scholar]
- 3.Gerstner HB, Huff JE. Clinical Toxicology of Mercury. J. Toxicol. and. Environ. Health. 1977;2:491–526. doi: 10.1080/15287397709529452. [DOI] [PubMed] [Google Scholar]
- 4.Clarkson TW. The toxicology of mercury. Crit. Rev. Clin. Lab. Sci. 1997;34(3):369–403. doi: 10.3109/10408369708998098. [DOI] [PubMed] [Google Scholar]
- 5.Risher JF, Murray HE, Prince GR. Organic mercury compounds: human exposure and its relevance to public health. Toxicol. Ind. Health. 2002;18(3):109–160. doi: 10.1191/0748233702th138oa. [DOI] [PubMed] [Google Scholar]
- 6.Silbergeld EK, Silva IA, Nyland JF. Mercury and autoimmunity: implications for occupational and environmental health. Toxicol Appl Pharmacol. 2005;207(2 Suppl):282–292. doi: 10.1016/j.taap.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 7.Queiroz MLS, Dantas DCM. T lymphocytes in mercury-exposed workers. Immunopharmacol. and. Immunotoxicol. 1997;19:499–510. doi: 10.3109/08923979709007671. [DOI] [PubMed] [Google Scholar]
- 8.Queiroz MLS, Dantas DCM. B lymphocytes in mercury-exposed workers. Pharmacology & Toxicology. 1997;81:130–133. doi: 10.1111/j.1600-0773.1997.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 9.Dantas DCM, Queiroz MLS. Immunoglobulin E and autoantibodies in mercury-exposed workers. Immunopharmacol. and. Immunotoxicol. 1997;19:383–392. doi: 10.3109/08923979709046983. [DOI] [PubMed] [Google Scholar]
- 10.Mayes MD. Epidemiologic studies of environmental agents and systemic autoimmune diseases. 107. Environ. Health Perspect. 1999;(Suppl 5):743–748. doi: 10.1289/ehp.99107s5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper GS, Parks CG, Treadwell EL, St Clair EW, Gilkeson GS, Dooley MA. Occupational risk factors for the development of systemic lupus erythematosus. J. Rheumatol. 2004;31(10):1928–1933. [PubMed] [Google Scholar]
- 12.Dahlgren J, Takhar H, Anderson-Mahoney P, Kotlerman J, Tarr J, Warshaw R. Cluster of systemic lupus erythematosus (SLE) associated with an oil field waste site: a cross sectional study. Environ. Health. 2007;6(1):8. doi: 10.1186/1476-069X-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hultman P, Taylor A, Yang JM, Pollard KM. The effect of xenobiotic exposure on spontaneous autoimmunity in (SWR x SJL)F1 hybrid mice. J. Toxicol. Environ. Health A. 2006;69(6):505–523. doi: 10.1080/15287390500354904. [DOI] [PubMed] [Google Scholar]
- 14.Pollard KM, Pearson DL, Hultman P, Hildebrandt B, Kono DH. Lupus-Prone Mice as Models to Study Xenobiotic-Induced Acceleration of Systemic Autoimmunity. Environ Health Perspect. 1999;107(Suppl 5):729–735. doi: 10.1289/ehp.99107s5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollard KM, Pearson DL, Hultman P, Deane TN, Lindh U, Kono DH. Xenobiotic acceleration of idiopathic systemic autoimmunity in lupus-prone bxsb mice. Environ. Health Perspect. 2001;109(1):27–33. doi: 10.1289/ehp.0110927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenden N, Rabbani H, Abedi-Valugerdi M. Analysis of mercury-induced immune activation in nonobese diabetic (NOD) mice. Clin. exp. Immunol. 2001;125(2):202–210. doi: 10.1046/j.1365-2249.2001.01580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Via CS, Nguyen P, Niculescu F, Papadimitriou J, Hoover D, Silbergeld EK. Low-dose exposure to inorganic mercury accelerates disease and mortality in acquired murine lupus. Environ. Health Perspect. 2003;111(10):1273–1277. doi: 10.1289/ehp.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansson M, Djerbi M, Rabbani H, Mellstedt H, Gharibdoost F, Hassan M, Depierre JW, Abedi-Valugerdi M. Exposure to mercuric chloride during the induction phase and after the onset of collagen-induced arthritis enhances immune/autoimmune responses and exacerbates the disease in DBA/1 mice. Immunology. 2005;114(3):428–437. doi: 10.1111/j.1365-2567.2005.02105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–299. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 20.Haase H, Engelhardt G, Hebel S, Rink L. Mercuric ions inhibit mitogen-activated protein kinase dephosphorylation by inducing reactive oxygen species. Toxicol. Appl. Pharmacol. 2011;250(1):78–86. doi: 10.1016/j.taap.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Eyrich B, Sickmann A, Zahedi RP. Catch me if you can: mass spectrometry-based phosphoproteomics and quantification strategies. Proteomics. 2011;11(4):554–570. doi: 10.1002/pmic.201000489. [DOI] [PubMed] [Google Scholar]
- 22.Rigbolt KT, Blagoev B. Quantitative phosphoproteomics to characterize signaling networks. Semin. Cell Dev. Biol. 2012;23(8):863–871. doi: 10.1016/j.semcdb.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Engholm-Keller K, Larsen MR. Technologies and challenges in large-scale phosphoproteomics. Proteomics. 2013;13(6):910–931. doi: 10.1002/pmic.201200484. [DOI] [PubMed] [Google Scholar]
- 24.Shu H, Chen S, Bi Q, Mumby M, Brekken DL. Identification of phosphoproteins and their phosphorylation sites in the WEHI-231 B lymphoma cell line. Mol. Cell Proteomics. 2004;3(3):279–286. doi: 10.1074/mcp.D300003-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.Cutillas PR, Geering B, Waterfield MD, Vanhaesebroeck B. Quantification of gel-separated proteins and their phosphorylation sites by LC-MS using unlabeled internal standards: analysis of phosphoprotein dynamics in a B cell lymphoma cell line. Mol. Cell Proteomics. 2005;4(8):1038–1051. doi: 10.1074/mcp.M500078-MCP200. [DOI] [PubMed] [Google Scholar]
- 26.Lemeer S, Heck AJ. The phosphoproteomics data explosion. Curr. Opin. Chem. Biol. 2009;13(4):414–420. doi: 10.1016/j.cbpa.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 27.Jones AM, Nuhse TS. Phosphoproteomics using iTRAQ. Methods Mol. Biol. 2011;779:287–302. doi: 10.1007/978-1-61779-264-9_17. [DOI] [PubMed] [Google Scholar]
- 28.Pimienta G, Chaerkady R, Pandey A. SILAC for global phosphoproteomic analysis. Methods Mol. Biol. 2009;527:107–16. x. doi: 10.1007/978-1-60327-834-8_9. [DOI] [PubMed] [Google Scholar]
- 29.Lundgren DH, Hwang SI, Wu L, Han DK. Role of spectral counting in quantitative proteomics. Expert. Rev. Proteomics. 2010;7(1):39–53. doi: 10.1586/epr.09.69. [DOI] [PubMed] [Google Scholar]
- 30.Neilson KA, Ali NA, Muralidharan S, Mirzaei M, Mariani M, Assadourian G, Lee A, van Sluyter SC, Haynes PA. Less label, more free: approaches in label-free quantitative mass spectrometry. Proteomics. 2011;11(4):535–553. doi: 10.1002/pmic.201000553. [DOI] [PubMed] [Google Scholar]
- 31.Pan X, Whitten DA, Wu M, Chan C, Wilkerson CG, Pestka JJ. Global protein phosphorylation dynamics during deoxynivalenol-induced ribotoxic stress response in the macrophage. Toxicol. Appl. Pharmacol. 2013;268(2):201–211. doi: 10.1016/j.taap.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002;74(20):5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 33.Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat. Biotechnol. 2006;24(10):1285–1292. doi: 10.1038/nbt1240. [DOI] [PubMed] [Google Scholar]
- 34.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31(13):3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taverner T, Karpievitch YV, Polpitiya AD, Brown JN, Dabney AR, Anderson GA, Smith RD. DanteR: an extensible R-based tool for quantitative analysis of omics data. Bioinformatics. 2012;28(18):2404–2406. doi: 10.1093/bioinformatics/bts449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev. Toxicol. 2006;36(8):609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 37.Wright VP, Reiser PJ, Clanton TL. Redox modulation of global phosphatase activity and protein phosphorylation in intact skeletal muscle. J. Physiol. 2009;587(Pt 23):5767–5781. doi: 10.1113/jphysiol.2009.178285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev. Toxicol. 2006;36(8):609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 39.Sommer D, Coleman S, Swanson SA, Stemmer PM. Differential susceptibilities of serine/threonine phosphatases to oxidative and nitrosative stress. Arch. Biochem. Biophys. 2002;404(2):271–278. doi: 10.1016/s0003-9861(02)00242-4. [DOI] [PubMed] [Google Scholar]
- 40.Bakalarski CE, Haas W, Dephoure NE, Gygi SP. The effects of mass accuracy, data acquisition speed, and search algorithm choice on peptide identification rates in phosphoproteomics. Anal. Bioanal. Chem. 2007;389(5):1409–1419. doi: 10.1007/s00216-007-1563-x. [DOI] [PubMed] [Google Scholar]
- 41.Huyer G, Liu S, Kelly J, Moffat J, Payette P, Kennedy B, Tsaprailis G, Gresser MJ, Ramachandran C. Mechanism of inhibition of protein tyrosine phosphatases by vanadate and pervanadate. J. Biol. Chem. 1997;272(2):843–851. doi: 10.1074/jbc.272.2.843. [DOI] [PubMed] [Google Scholar]
- 42.Que S, Wang Y, Chen P, Tang YR, Zhang Z, He H. Evaluation of protein phosphorylation site predictors. Protein Pept. Lett. 2010;17(1):64–69. doi: 10.2174/092986610789909412. [DOI] [PubMed] [Google Scholar]
- 43.Liu BA, Nash PD. Evolution of SH2 domains and phosphotyrosine signalling networks. Philos. Trans. R. Soc. Lond B Biol. Sci. 2012;367(1602):2556–2573. doi: 10.1098/rstb.2012.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodnow CC. Balancing imunity and tolerance: Deleting and tuning lymphocyte repertoires. Proc. Natl. Acac. Sci. USA. 1996;93:2264–2271. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grimaldi CM, Michael DJ, Diamond B. Cutting edge: expansion and activation of a population of autoreactive marginal zone B cells in a model of estrogen induced lupus. J. Immunol. 2001;167(4):1886–1890. doi: 10.4049/jimmunol.167.4.1886. [DOI] [PubMed] [Google Scholar]
- 47.Grimaldi CM, Hicks R, Diamond B. B cell selection and susceptibility to autoimmunity. J. Immunol. 2005;174(4):1775–1781. doi: 10.4049/jimmunol.174.4.1775. [DOI] [PubMed] [Google Scholar]
- 48.McCabe MJ, Jr., Eckles KG, Langdon M, Clarkson TW, Whitekus MJ, Rosenspire AJ. Attenuation of CD95-induced apoptosis by inorganic mercury: caspase-3 is not a direct target of low levels of Hg2+. Toxicol. Lett. 2005;155(1):161–170. doi: 10.1016/j.toxlet.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 49.Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 2005;6(4):318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- 50.Rahman SM, Pu MY, Hamaguchi M, Iwamoto T, Isobe K, Nakashima I. Redox-linked ligand-independent cell surface triggering for extensive protein tyrosine phosphorylation. FEBS Lett. 1993;317(1-2):35–38. doi: 10.1016/0014-5793(93)81486-j. [DOI] [PubMed] [Google Scholar]
- 51.Nakashima I, Pu MY, Nishizaki A, Rosila I, Ma L, Katano Y, Ohkusu K, Rahman SMJ, Isobe K, Hamaguchi M, Saga K. Redox mechanism as alteranative to ligand binding for receptor activation delivering disregulated cellular signals. J. Immunol. 1994;152:1064–1071. [PubMed] [Google Scholar]
- 52.Nita-Lazar A. Quantitative analysis of phosphorylation-based protein signaling networks in the immune system by mass spectrometry. Wiley. Interdiscip. Rev. Syst. Biol. Med. 2011;3(3):368–376. doi: 10.1002/wsbm.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vizcaino JA, Cote RG, Csordas A, Dianes JA, Fabregat A, Foster JM, Griss J, Alpi E, Birim M, Contell J, O'Kelly G, Schoenegger A, Ovelleiro D, Perez Riverol Y, Reisinger F, Rios D, Wang R, Hermjakob H. The PRoteomics IDEntifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 2013;41(Database issue):D1063–D1069. doi: 10.1093/nar/gks1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.