Abstract
Background
Chronic heart failure (CHF) is a serious, common condition associated with frequent hospitalisation. Several different disease management interventions (clinical service organisation interventions) for patients with CHF have been proposed.
Objectives
To assess the effectiveness of disease management interventions for patients with CHF.
Search methods
We searched: Cochrane CENTRAL Register of Controlled Trials (to June 2003); MEDLINE (January 1966 to July 2003); EMBASE (January 1980 to July 2003); CINAHL (January 1982 to July 2003); AMED (January 1985 to July 2003); Science Citation Index Expanded (searched January 1981 to March 2001); SIGLE (January 1980 to July 2003); DARE (July 2003); National Research Register (July 2003); NHS Economic Evaluations Database (March 2001); reference lists of articles and asked experts in the field.
Selection criteria
Randomised controlled trials comparing disease management interventions specifically directed at patients with CHF to usual care.
Data collection and analysis
At least two reviewers independently extracted data information and assessed study quality. Study authors were contacted for further information where necessary.
Main results
Sixteen trials involving 1,627 people were included. We classified the interventions into three models: multidisciplinary interventions (a holistic approach bridging the gap between hospital admission and discharge home delivered by a team); case management interventions (intense monitoring of patients following discharge often involving telephone follow up and home visits); and clinic interventions (follow up in a CHF clinic). There was considerable overlap within these categories, however the components, intensity and duration of the interventions varied.
Case management interventions tended to be associated with reduced all cause mortality but these findings were not statistically significant (odds ratio 0.86, 95% confidence interval 0.67 to 1.10, P = 0.23), although the evidence was stronger when analysis was limited to the better quality studies (odds ratio 0.68, 95% confidence interval 0.46 to 0.98, P = 0.04). There was weak evidence that case management interventions may be associated with a reduction in admissions for heart failure. It is unclear what the effective components of the case management interventions are.
The single RCT of a multidisciplinary intervention showed reduced heart-failure related re-admissions in the short term. At present there is little available evidence to support clinic based interventions.
Authors’ conclusions
The data from this review are insufficient for forming recommendations. Further research should include adequately powered, multicentre studies. Future studies should also investigate the effect of interventions on patients’ and carers’ quality of life, their satisfaction with the interventions and cost effectiveness.
Medical Subject Headings (MeSH): Case Management [*organization & administration], Chronic Disease, Heart Failure [*therapy], Randomized Controlled Trials as Topic
MeSH check words: Humans
BACKGROUND
Chronic heart failure (CHF) is a serious and increasingly common condition (Cleland 1999; Cowie 1997; Eriksson 1995) with a crude prevalence of 3 to 20 per 1000 in the general population (Cowie 1999). Both the incidence and prevalence of CHF increase with age, from around one per cent of those aged 50-59 years to 10 per cent of those aged 80-89 years (Kannel 1991) and most patients with heart failure are elderly. In Scotland the mean age at first hospital admission for CHF is 74 years (Cleland 1999) and in the United States half of all patients over 65 years admitted with CHF are over 80 years old (Havranek 2002). The condition carries a substantial risk of death - in recent community studies between a quarter and a third of patients were dead one year after the onset of heart failure (Cowie 2000; Levy 2002), and around two thirds of men and half of women were dead after five years (Levy 2002). In a study of Scottish data the median survival time after a first hospital admission with CHF was sixteen months and the five year survival rate was 25% - worse than that for all common malignancies except lung and ovarian cancer (Stewart 2001b). A Canadian population based study of survival after a first hospital admission for heart failure reported a case fatality rate of 31% at one year follow up (Jong 2002). In addition to the risk of death the condition has a profound impact on patients’ quality of life (Stewart 1989).
Hospital admissions for heart failure have steadily increased and heart failure is now one of the most common reasons for admission in older people (AHA 2004; Cleland 1999; McMurray 1993). It has been estimated that in 2000 1.9% of the total budget of the National Health Service (£905 million) was spent on patients with heart failure and most of this cost was incurred by hospital admissions (Stewart 2002a). A community study from England found 55% of patients in primary care being treated with loop diuretics and with a clinical diagnosis CHF had an acute admission to hospital with heart failure (Clarke 1994). Early hospital readmission in patients with heart failure is extremely common. In Connecticut, USA, between 1991 and 1994, 44% of all patients admitted for congestive heart failure were re-admitted (all causes) within six months (Krumholz 1997). In the recent EuroHeart Failure survey, which included 24 countries, 24% of patients admitted with confirmed or suspected heart failure were readmitted to hospital within 12 weeks - heart failure was the principal cause of readmission (20% of readmissions) and contributed to a further 16% of readmissions (Cleland 2003). Studies suggest that many early re-admissions for heart failure are preventable (Feenstra 1998; Michalsen 1998; Vinson 1990).
Drug therapy is the mainstay of treatment for CHF, although invasive procedures are indicted for some patients, and patients are usually managed with a combination of medications and lifestyle advice (NICE 2003). The management of patients with heart failure has been described as evolving from the traditional model with its emphasis on crisis intervention towards more proactive, preventative disease management models. These emerging care models offer “aggressive care” in hospital, home or clinic (Riegel 2001). In view of the importance of heart failure both to patients and to health services as a whole, a systematic review of specific interventions aimed at reducing hospital re-admissions in heart failure is needed to help inform health care professionals in the provision of more effective care for these patients.
OBJECTIVES
Primary objective
To assess systematically the effects of different clinical service interventions, which are not primarily educational in focus, in preventing death and/or hospital re-admission in patients who have previously been admitted to secondary care with a diagnosis of heart failure. (Wherever possible examining event free survival: that is survival without hospital re-admission).
Secondary objective
To assess the effects of the different clinical service interventions in terms of other outcomes that may have been reported such as hospital bed days, health related quality of life and cost.
METHODS
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials were included in the analyses.
Types of participants
Adults who had at least one admission to secondary care with a diagnosis of heart failure were the focus of this review. Studies dealing principally with patients with cardiac disorders other than heart failure, or with heart failure arising from congenital heart disease and/or valvular heart disease, were excluded.
Types of interventions
Clinical service interventions were defined as inpatient, outpatient or community based interventions or packages of care, excluding the simple prescription or administration of a pharmaceutical agent(s), which are applied to patients with heart failure and their relatives or carers. These interventions included enhanced or novel service provision for patients with heart failure. Interventions that were primarily educational in focus were not included in this review. Interventions that included an educational component as part of a broader programme of enhanced service provision were included in this review. These interventions were compared with ‘usual care’ for this patient group.
Types of outcome measures
Primary outcomes
deaths (all cause and heart failure related);
re-admission to secondary care;
total number of re-admissions and number of unplanned re-admissions;
total hospital bed days (all cause and heart failure related);
length of time between index hospital discharge and unplanned re-admission;
event free survival (with an event defined as death or hospital re-admission).
We intended to examine re-admissions at fixed time intervals from discharge if possible.
Secondary outcomes
health related quality of life;
cost analyses.
Search methods for identification of studies
The search strategy was developed before the Heart Cochrane Review Group search strategy was published and our search strategy was wider since we originally considered including non-randomised, prospective studies with concurrent control groups in secondary analyses. (This idea was abandoned because we had difficulty in identifying studies which met our quality criteria and the results of the very few non-randomised studies we considered including did not influence the conclusions of this review). The searches for the individual databases are shown in the additional studies table (Table 1). No language restrictions were applied.
Our search consisted of the following steps:
-
(1)The following electronic databases were searched:
- Cochrane CENTAL Register of Controlled Trials (CENTRAL), The Cochrane Library, Issue 2 2003;
- MEDLINE January 1966 to July 2003;
- EMBASE January 1980 to July 2003;
- CINAHL (Cumulative Index to Nursing and Allied Health Literature) January 1982 to July 2003;
- AMED (Allied and Alternative Medicine Database, covers occupational therapy, physiotherapy and complementary medicine) January 1985 to July 2003;
- Science Citation Index Expanded searched January 1981 to March 2001 (forward and backwards search, see below);
- SIGLE Jan 1980 to July 2003;
- Database of Abstracts of Reviews of Effects (DARE) to July 2003;
- National Research Register to July 2003;
- NHS Economic Evaluations Database to March 2001;
- Cardio-Vascular Disease (CVD) Trials Registry at McMaster University (entire database searched on 7/2/2001);
- Chartered Society of Physiotherapy (CSP) Library Catalogue to June 2001.
-
(2)
Citation tracking: reference lists of retrieved articles and published reviews on the topic were retrieved. In addition to a backward search of the Science Citation Index using key words (see below) we also conducted a forward search of articles using the five earliest eligible studies identified from electronic database searching.
-
(3)
Personal communication with the principal investigators of the identified RCTs and with national and international experts in the field.
Data collection and analysis
-
(1)
CENTRAL was searched by the Cochrane Heart Group. All other electronic searches were conducted by two members of the group working independently. A librarian with extensive expertise in electronic databases provided advice on searching;
-
(2)
Group training was conducted on the first 100 references retrieved from searches of two different databases to ensure that the group had a consistent approach to assessing titles and abstracts;
-
(3)
The title and abstract of each reference retrieved was assessed by two members working independently. Titles and abstracts of non-English language papers were translated into English;
-
(4)
The full texts of all potentially eligible papers were obtained and assessed for eligibility by two members of the group working independently. Non English language papers which appeared to be eligible for inclusion on the basis of the translation of title and abstract were fully translated in to English;
-
(5)
Any disagreements about eligibility were resolved by discussion between at least three members of the group;
-
(6)
A data abstraction form was developed and the group worked together on several papers to ensure that members had a consistent approach to data abstraction;
-
(7)
All eligible papers were formally abstracted by at least two members of the group working independently and using the data collection form. Any disagreements were resolved by discussion with another member of the group;
-
(8)
Where we were unclear about issues arising from their published papers we attempted to contact the authors for clarification.
Assessing the methodological quality and external validity of the trials
The quality of the studies was assessed in terms of allocation concealment and, not specified in our protocol but added in order to enhance our understand of the studies, we also considered the criteria for quality assessment of RCTs developed by Verhagen (Verhagen 1998). (We excluded two items “was the patient blinded?/masked” and “was the care provider masked?”, since these make less sense in the context of the type of interventions under study).
The quality items considered were:
-
(1)Treatment allocation
-
(a)Was a method of randomisation performed?
-
(b)Was the treatment allocation concealed?
-
(a)
-
(2)
Were the groups similar at baseline regarding the most important prognostic indicators?
-
(3)
Were the eligibility criteria specified?
-
(4)
Was the outcome assessor masked?
-
(5)
Were point estimates and measures of variability presented for the primary outcome measures?
-
(6)
Did the analysis include an intention to treat analysis?
We also commented on the risk of attrition bias. Statistical power and generalisability refer to the external validity of studies and we also commented on these. All included studies were examined by two medical statisticians working independently.
Categorising the interventions
Riegel has proposed three types of heart failure disease management models and, although we did not mention any categorisation in our protocol, we have used her typology to group the different interventions for synthesis (Riegel 2001). The models are described as follows:
Multidisciplinary models
Multidisciplinary models offer a holistic approach to the individuals’ medical, psychosocial, behavioural and financial circumstances and typically involve several different professions working in collaboration. “The gap between hospitalisation, other health care delivery systems (e.g. skilled nursing facilities, hospice) and home is bridged by a team of individuals knowledgeable about heart failure and committed to patient care.”
Case management models
Case management models consist of intense monitoring of the patients following discharge from hospital, this is usually done by a nurse and typically involves home visits and/or telephone calls.
Clinic models
Clinic models involve outpatient clinics for heart failure, they are usually run by cardiologists with a special interest in heart failure or by specialist nurses using agreed protocols to manage medication. Results of the individual studies were initially combined in a narrative review, weighted according to the methodological quality of each. Where possible and appropriate, the trial results were combined statistically using meta-analytic methods.
Data analysis
Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for dichotomous variables, such as death. In the absence of significant heterogeneity, using the Cochrane Q statistic (p>0.1), summary ORs and 95% CIs were calculated using a fixed-effects meta-analysis.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
The electronic searches retrieved a number of studies examining the effect of interventions directed at populations of older people and not exclusively aimed at patients with heart failure. We have excluded these studies from this review (See Excluded Studies Table). Inclusion of studies examining the effect of these sort of “generic” interventions would have necessitated a different search strategy.
Our search strategy identified 28,046 papers including very many duplicates and a number of reviews and guidelines. We excluded 27,840 references by removing duplicates and after screening titles and abstracts. Two hundred and six papers, including major review articles and guidelines, were retrieved. Examination of the full papers led to the exclusion of a further 185 papers. Excluded studies which relate to the area reviewed are described in the Excluded Studies Table.
We identified 21 publications for inclusion in the review; these described 16 individual RCT studies and 15 different interventions. Two of the RCTs identified were feasibility or pilot studies (Ekman 1998; Rich 1993), one (Rich 1993) informed a much larger study (Rich 1995) which is also included in the review. Control patients received unrestricted ‘usual’ or ‘routine’ care in all the studies except one where both control and intervention patients received a programme of ‘optimised’ medical care during the index hospitalisation (McDonald 2002).
All the included studies were conducted at a single centre with the exception of one which involved two centres (Kasper 2002). All the studies were led by professionals from secondary or tertiary care. As determined by scrutiny of the published accounts, none of the 15 different interventions were delivered in exactly the same way by the same type of personnel, although some were very similar and all the interventions had overlapping content (see Table 2) The interventions varied in site, intensity and duration (see Table 2, and Table of characteristics of included studies). Length of follow up ranged from 12 weeks to one year.
Content of the interventions as described in the published reports
Telephone follow up
Ten interventions included scheduled, pro active telephone follow up of patients at home and a two further studies involved a single telephone call following hospital discharge .
Education
Education aimed at patients, and in some cases carers, appears to have been a major component in at least twelve of the interventions. The education typically covered the diagnosis, symptoms and treatment of heart failure and when to seek expert help.
Self management
Many of the interventions actively sought to promote better patient self management and patients were sometimes given heart failure diaries or notebooks to aid self management.
Weight monitoring
Daily or regular weight monitoring, or the importance of weight monitoring, was mentioned in nine of the interventions, patients in these studies were often given charts or diaries in which to log their weights and in two cases some patients were supplied with weigh scales.
Sodium restriction and/or dietary advice
This was mentioned in seven interventions and ranged from a dietician’s visit and an individualised 1.5-2.0 g per day sodium diet to a list of dietary recommendations.
Exercise recommendations
These were specifically mentioned in six of the studies.
Medication review
Only two of the interventions specifically mentioned a review of the patients’ medications, in one this was conducted by a geriatric cardiologist and in the other by a hospital pharmacist.
Social support and psychological support
Social workers assessed patients’ needs in two interventions, outpatient support groups featured in one intervention and one study stated that the heart failure specialist nurse gave patients psychological support.
Table 2 lists the components of the interventions as described in the published papers against the studies.
Inclusion and Exclusion Criteria
All the studies differed in their inclusion and exclusion criteria. The age range for patients included in the studies varied from over 18 years (Jaarsma 2000; McDonald 2002) to over 70 years (Rich 1995); one study had an upper age limit of 84 years (Cline 1998). All the studies identified patients during an index hospital admission for CHF but several reports did not specify the criteria used for identifying CHF. One study required patients to have had at least one admission for acute heart failure prior to the index admission (Stewart 1999a) and three other interventions were targeted at patients the researchers considered to be at high risk for re-admission (Kasper 2002; Laramee 2003; Rich 1995). One study required that patients would be discharged home with nursing care (Harrison 2002). Only one study specifically excluded patients with diastolic heart failure (Blue 2001).
Five of the studies mentioned excluding patients with valvular heart disease requiring surgery (Doughty 2002; Jaarsma 2000; Kasper 2002; McDonald 2002; Stewart 1999a). Several studies specifically excluded CHF associated with acute myocardial infarction (Blue 2001; Ekman 1998; Kasper 2002; McDonald 2002) although CHF precipitated by acute MI was one of four independent risk factors in the inclusion criteria in one study (Rich 1995). The presence of serious co-morbidity or other terminal illness was a common exclusion criterion (Blue 2001; Cline 1998; Ekman 1998; Harrison 2002; Jaarsma 2000; Krumholz 2002; Laramee 2003; McDonald 2002; Rainville 1999; Riegel 2002) and most of the studies excluded patients discharged to long term care facilities such as nursing homes (Blue 2001; Ekman 1998; Harrison 2002, Jaarsma 2000; Kasper 2002; Krumholz 2002; Larame 2003; McDonald 2002; Rainville 1999; Rich 1995; Riegel 2002).
The patients enrolled in the studies
The mean or median age of the patients involved in the interventions lay between 70 and 80 years. However they were younger in Kasper’s study (median age 63.5 years, range 25-88) and the participants in Capomolla’s study were particularly young compared to the other studies (mean age 56 years, SD 10). The proportion of male study subjects varied from 86% (Capomolla 2002) to 23% (Rich 1995). The proportion of patients from different ethnic groups was rarely stated. Where it was reported this ranged from 45% white (Rich 1995) to 77% ‘European’ (Doughty 2002).
Categorising the interventions
The types of personnel involved in the interventions differed but specialist nurses were common to all studies, although the level of their involvement varied. We used Riegel’s classification (Riegel 2002, see Methods) to group the interventions based on the content and nature of the interventions as they were described in the papers. In practice there appears to be considerable overlap between these disease management models and it was not always easy to classify them, Table 2 summarises some of the similarities and differences between the interventions. One intervention involved a day hospital heart failure management programme (Capomolla 2002) and was difficult to categorise. We considered that the remaining interventions fell predominantly into the following groups:
One reflected a multidisciplinary approach (Rich 1993; Rich 1995).
Eleven RCTs appeared to involve variations on the case management approach (Cline 1998; Rainville 1999; Stewart 1999a; Jaarsma 2000; Blue 2001; Harrison 2002; Kasper 2002; Krumholz 2002; McDonald 2002; Riegel 2002; Laramee 2003). Two of these interventions were largely educational (Jaarsma 2000; Krumholz 2002), and three involved a combination of case management with follow up in a heart failure clinic (Cline 1998; Kasper 2002; McDonald 2002).
Two represented clinic models (Ekman 1998; Doughty 2002).
Risk of bias in included studies
We have noted in the Table results of included studies (Table 3) where there were concerns about the suitability or clarity of the particular statistical tests reported in the papers. Allocation concealment had been practiced in seven of the 16 studies (Blue 2001; Ekman 1998; Harrison 2002; Kasper 2002; McDonald 2002; Rich 1995; Stewart 1999a) and the outcome assessor was masked in five (Blue 2001; Harrison 2002; Kasper 2002; Krumholz 2002; Stewart 1999a) (see Table characteristics of included studies). Results of methodological quality assessment of the included RCTs using the Delphi criteria are shown in the Delphi Table (Table 4). Only one study met all the Delphi criteria we used (Stewart 1999a). Eight other studies (Blue 2001; Ekman 1998; Harrison 2002; Kasper 2002; Krumholz 2002; McDonald 2002; Rich 1993; Rich 1995) appeared to be of at least moderate quality using the Delphi criteria, although in half of these the outcome assessor was not masked. Three studies appeared to be of lower quality using these criteria (Jaarsma 2000; Laramee 2003; Rainville 1999) and there was insufficient information to assess the quality of the remaining four studies (Capomolla 2002; Cline 1998; Doughty 2002; Riegel 2002).
Statistical consideration of the studies
Even excluding the pilot and feasibility studies, most of the studies in the review had fairly small sample sizes. A power calculation shows that if the proportion of patients who are event free in the intervention arm is 0.3 and 0.5 in the control arm then 134 patients are needed in both arms to give 80% power of detecting the difference with the probability of a type 1 error of 0.05. Few of the included studies had samples of this size. There are a few cases where significant results are reported in very small studies (e.g. (Rainville) 17 in each arm; (Krumholz) 48 in each arm; (McDonald) 51 and 47 in the two arms). Here the studies are not underpowered because a significant result has been found, but when interpreting and attempting to synthesise these results we must be aware of the possibility of publication bias. Comments on statistical considerations for the individual studies are given in Table 3.
Effects of interventions
The Table of results of included studies (Table 3) documents results of primary and secondary endpoints and includes a comment on the statistical analyses used in each individual paper.
Synthesis of the findings from the included studies
Because of our concerns about the analysis of one study (Riegel 2001, see Table 3) we have excluded this study from the synthesis of all the outcomes except mortality. We have presented the results of Capomolla’s study separately because of the unique characteristics of both the intervention and the patients it was directed at (see Characteristics of Included Studies Table).
Mortality
All cause deaths
Thirteen of the 16 studies provided information on all cause mortality, none reported a significant difference in all cause mortality between intervention and control patients. Meta-analysis of the 10 case management interventions with data on mortality revealed a non-significant tendency for these interventions to be associated with reduced mortality, odds ratio 0.86 (95% confidence interval 0.67 to 1.10, P = 0.23) (Figure 01.01), however it must be emphasised that these interventions differed in content and that duration of the intervention and the length of follow up varied (see Table 5 and characteristics of included studies table). (We have ordered the studies by length of follow up in Figure 01.0, We rejected the idea of conducting separate analyses by duration of follow up because there would be very few studies in each group). When the meta-analysis was limited to the four case management studies with allocation concealment the odds ratio approached significance, odds ratio 0.69 (95% confidence interval 0.46 to 1.03, P = 0.07) (Figure 01.02) and when it was limited to those studies considered to be of at least moderate quality using the Delphi criteria there was a significant tendency for improved survival with the intervention, odds ratio 0.68 (95% confidence interval 0.46 to 0.98, P = 0.04) (Figure 01.03). Again it must be noted that the content and duration of these interventions, and the length of follow up varied. Our meta-analysis of the clinic intervention RCTs must be interpreted with extreme caution since there were only two studies and they had different follow up periods; no evidence of an effect on mortality was seen, odds ratio 0.95 (95% confidence interval 0.57 to 1.57, P = 0.83) (Figure 02.01). The single RCT of a multidisciplinary intervention found a greater proportion of deaths in the intervention group compared to the control group across the follow up period but the difference was not significant (Table 3).
Heart failure related mortality
Only one study reported on heart failure or cardiac related mortality: Capomolla’s study (Capomolla 2002) of a day hospital based heart failure management programme reported a highly significant reduction in cardiac related deaths in the intervention group. However total deaths were not reported, it is not clear how cardiac related deaths were identified, and the study population appears to be highly selected so the generalisability of this finding is unclear.
Event free survival
Information on event free survival (survival without all cause re-admission or death) was provided for nine of the 16 included RCTs. Event free survival was reported in a variety of ways. The most common way was as comparisons of proportions of patients who had experienced death or re-admission at different time points, sometimes survival curves and log-rank tests, hazard ratios or Cox’s proportional hazards regression analyses were presented. It was not feasible to conduct a statistical meta-analysis on these results.
Seven case management interventions reported event free survival, usually this meant the avoidance of both death from any cause and hospital readmission for heart failure. At three months follow up one moderate quality study reported a significant difference favouring case management (4 vs. 12, P = 0.04, Fisher’s exact test) (McDonald 2002). A study of unclear quality which considered death or readmission (probably all cause readmission) at three months reported no significant differences (Cline 1998). At six months follow up one high quality case management study (Stewart 1999a) found more patients survived without an unplanned readmission to hospital amongst the intervention group than the control group 51% vs. 38%, P = 0.04, (95% confidence intervals not given) whilst one moderate quality study reported no difference (P = 0.12 log-rank test) (Kasper 2002). At 12 months follow up two moderate quality studies reported hazard ratios for event free survival which favoured the case management intervention; Blue 2001, 31 vs. 43, hazard ratio 0.61 (95% confidence interval 0.38 to 0.96, P = 0.03); (Krumholz 2002, hazard ratio 0.5 (95% confidence interval 0.29 to 0.09, P = 0.02) as did another very small study judged to be of lower quality (P < 0.01 log rank test) (Rainville 1999). Cline reported no significant differences on event free survival at 12 months (56 patients (70%) vs. 79 (72%), Cline 1998).
Neither of the two studies of clinic interventions reported a significant difference in event free survival between intervention and control groups (Ekman 1998, moderate quality, 30 patients (30%) vs. 25 (32%) surviving at six months; Doughty 2002, unknown quality, event free survival at 12 months P = 0.33 kaplan meier survival curves). Both these studies may have lacked sufficient power Doughty’s was terminated early and Ekman’s was a smaller feasibility study (see Table 3). The single, large RCT of a multidisciplinary intervention found no significant difference in event free survival at three months between intervention and control groups (Rich 1995), this was the study’s primary outcome and the study was adequately powered.
Only one paper (McDonald 2002) reported on survival without heart failure related hospital admission. This found a significant reduction in the case managed group but the outcome assessors were not masked and it is not clear how heart failure related re-admissions were defined or identified.
Re-admissions to secondary care
Unplanned re-admissions
There is evidence from one high quality trial (Stewart 1999a) that case management may reduce the frequency of unplanned re-admissions (for all causes) at six months (mean re-admissions per month 0.14 (95% confidence interval 0.10 to 0.18); vs. 0.34 (95% confidence interval 0.19-0.49, P = 0.03, test not clear). This effect appears to have been sustained to 18 months; group mean re-admissions per month 0.15 (95% CI 0.11 to 0.19); vs. 0.37 (95% CI 0.19 to 0.55, P = 0.053). However it is not clear how unplanned re-admissions were defined or identified although outcome assessors were masked. None of the other included case management studies reported the frequency of unplanned re-admissions.
All cause re-admissions
Eleven of the 16 included studies provided some useable information on all cause hospital re-admissions. Information on all cause re-admissions was presented in a variety of ways by the individual studies (see Table 3) and it was not possible to perform any meaningful meta-analyses on these results. Seven of the eleven case management studies reported on all cause readmissions in some way at three (Harrison 2002; Laramee 2003), nine (Jaarsma 2000) and 12 months follow up (Blue 2001; Cline 1998; Krumholz 2002; Rainville 1999). Blue (Blue 2001) reported no difference in the number of patients admitted to hospital for all causes between the intervention and control groups but did report a reduction in the average number of admissions per month in the intervention group; hazard ratio 0.71 (95% confidence intervals 0.54 to 0.94, P = 0.02). In all but one of the other case management studies there appear to have been fewer readmissions in the case managed patients but the differences were not statistically significant in any of the studies.
Only one of the clinic studies reported on all cause readmissions: in Cline’s study there were fewer readmissions in the clinic managed group compared to the controls (re-admissions per patient per year 1.37 vs. 1.84, method of calculation not given, rate difference = 0.47 per patient per year (95% confidence interval 0.16 to 0.78) (Cline 1998). At three months follow up Rich found a significant reduction in the total number of all cause re-admissions in the multidisciplinary management group; total number of readmissions in 90 days 53 vs. 90, P = 0.02 Wilcoxon rank-sum test, patients with at least one re-admission in 90 days 41 (28.9%) vs. 59 (42.1%), absolute difference 13.2% (95% confidence interval 2.1 to 24.3, P = 0.03) but no significant difference in readmission rates after nine months (Rich 1995).
Capomolla (Capomolla 2002) noted a highly significant reduction in hospital readmissions in his intervention group (total number of hospital readmissions at mean 12 (SD 3) months follow up: 13 vs. 78, P<0.00001) but the generalisability and quality of this study are very unclear. It is also not clear if these are all cause readmissions or readmissions for haemodynamic instability.
Heart failure related re-admissions
Nine studies attempted to distinguish between heart failure, or cardiac, related events and events which were not related to heart failure or a cardiac illness. Such distinctions were necessary to determine the primary outcomes of some studies. Despite this only two RCTs provided any details in their publications on how they adjudicated whether events were heart failure related or not (Blue 2001; Kasper 2002) and none of the studies provided any information on the validation of these categorisations.
Only seven of the eleven case management studies reported the number of patients experiencing at least one hospital readmission for heart failure during follow up. Meta-analysis of these results suggests that case management may be associated with a reduction in heat failure readmissions during follow up; odds ratio 0.52 (95% confidence interval 0.39 to 0.70) (Figure 01.04). However it must be emphasised that these studies differed in their components, their duration, the length of follow up and their quality. Moreover the outcome assessor was masked in only three of these studies.
One clinic intervention study reported no differences in the number of hospital readmissions for heart failure or the number of patients experiencing a readmission at 12 months (Cline 1998). Rich (Rich 1995) reported significantly fewer heart failure related re-admissions in the multidisciplinary care group compared to the control group at three months but not at nine months.
Days spent in hospital during re-admissions
Eight studies (including Riegel 2002) reported on days spent in hospital during readmission. Stewart’s study of case management explored unplanned days in hospital and found a reduction in the intervention group at both at six months and 18 months; results at six months: 460 days vs. 1174, event rates per month 0.9 (95% confidence interval 0.6 to 1.2), vs. 2.9 (95% confidence interval 1.9 to 3.9, P = 0.01). However at six months there were similar proportions of unplanned re-admissions associated with a primary diagnosis of heart failure in each group (34 (50%) intervention vs. 58 (49%) controls) (Stewart 1999a). None of the other four case management studies reporting this outcome found a difference in all cause hospital bed days during follow up. Of the two moderate quality studies that examined days spent in hospital for readmissions associated with heart failure at 12 months, Blue 2001 reported significantly fewer bed days in the case managed groups (mean days spent in hospital with worsening HF 3.43, SD 12.2 vs. 7.46, SD 16.6 P = 0.005), whilst the second, Krumholz 2002 reported a reduction in bed days for cardiovascular readmissions including heart failure (mean days 6.3, SD 9.2, vs. 12.3, SD 14.3, P=0.03 test not given) but not for heart failure readmissions alone (mean days 4.1, SD 6.4 vs. 7.6, 12.1, P = 0.1, not significant).
Multidisciplinary management may also lead to a reduction in hospital bed days in the first 90 days after discharge: Rich 1995 found a significant reduction in total unplanned hospital bed days at six months in the intervention group compared to the control group. There is no evidence from the two studies to date that clinic models are associated with any reduction in days spent in hospital during follow up (Doughty 2002; Ekman 1998).
Length of time between index hospital discharge and unplanned re-admission
Three studies reported on the time between discharge and re-admission. Cline (Cline 1998, case management intervention, unclear quality) reported a significant increase in the mean length of time to re-admission in the intervention group in survivors at one year of follow up. Rainville’s extremely small case management study reported that time to re-admission or death was longer in the intervention group.
Doughty (Doughty 2002) noted no significant difference in mean time to re-admission in the clinic managed group compared to the control group.
Health Related Quality of Life
Health related quality of life (HRQL) was the principal outcome of two case management studies (Harrison 2002; Jaarsma 2000) and was mentioned as a secondary outcome in six other studies.
Harrison’s case management study (Harrison 2002, moderate quality) was powered to be able to detect a clinically significant difference in the Minnesota Living with Heart Failure Questionnaire (MLHFQ), she found significant improvements in the total MLHFQ score in the intervention group compared to the control group at six and 12 weeks follow up. Jaarsma’s largely educational case management intervention study (Jaarsma 2000) suffered severe attrition and was assessed to be of lower quality - no difference in HRQL between intervention and control groups was noted. In a randomly selected sub-sample of 68 patients Stewart found a statistically significant difference in change in MLHFQ favouring the intervention group in survivors at three months but not at six months (Stewart 1999a). A fourth case management intervention study (Kasper 2002) found a clinically significant improvement in MLHFQ scores after six months follow up in intervention patients compared to controls. Cline reported no difference in Quality of Life in Heart Failure Questionnaire scores at 12 months between intervention and control groups (Cline 1998) and McDonald 2002 found no improvement in HRQL at three months follow up but the method of measurement was not described.
Rich noted greater improvements in quality of life using the Chronic Heart Failure Questionnaire in those who received his multidisciplinary intervention amongst a subset of 126 patients; it is not known how these patients were selected nor how similar the two groups were at baseline, nor is it clear whether this difference is clinically important, although this seems likely.
Only one study of a clinic model intervention reported HRQL (Doughty 2002). There was no difference in MLHFQ total scores between intervention and control patients at one year, although the physical score showed a significantly greater improvement in the clinic managed patients compared to the control group. Capomolla measured quality of life in his day hospital managed patients compared to his control group using the time trade off method and found that the intervention group had significantly higher quality of life (see Table 3).
Cost Analyses
Only one study included a formal economic evaluation (Capomolla 2002), this report of a day hospital based heart failure programme intervention found the intervention saved $1,068 with every quality adjusted life year (QALY) gained with the intervention (see Table 3). Six of the other studies presented some cost data although the type of cost data, and the way it was presented, varied (Cline 1998; Kasper 2002; Krumholz 2002; Laramee 2003; Rich 1995; Stewart 1999a). No studies reported significant differences between intervention and control groups in the costs examined however all but one study (Kasper) found that the type of cost they reported was lower in the intervention group.
Adverse Events
None of the studies noted any adverse events arising from their interventions.
Generalisability of the results
To estimate the generalisability of results to all patients with heart failure admitted to hospital we considered the proportion of patients who were eligible for the interventions out of those screened and the proportion of eligible patients who were entered into the trials. These data were not always available. Two different interventions deliberately targeted patients considered to be at high risk of re-admission (Kasper 2002; Rich 1993; Rich 1995). In Rich’s 1995 multidisciplinary study 70% of patients who fulfilled his diagnostic criteria for CHF were considered to be at moderate or high risk of re-admission. However, 57% of these subjects were excluded and only 31% of the eligible patients were included in the study (22% of all the patients with CHF). In Kasper’s case management study, 67% of heart failure patients screened were considered to be at high risk of re-admission; 70% of these had one or more exclusion criteria and 20% of the eligible patients participated in the study (14% of all the patients with CHF). Ekman’s feasibility study (Ekman 1998) found that only 17% of 1058 consecutively screened subjects with a diagnosis of CHF or cardiomyopathy met the study eligibility criteria and only 13% of the screened patients participated in the study. In the other studies the proportion of patients thought to have heart failure on admission and eligible for the study varied between 32% (Rainville 1999) and 77% (Laramee 2003) and the proportion of potentially eligible patients who participated varied between 33% (Jaarsma 2000) and 80% (McDonald 2002).
DISCUSSION
This review systematically evaluated 15 different disease management interventions targeted at patients who have already experienced one hospital admission for heart failure. We recognise that high quality and definitive evidence for service delivery interventions such as, disease management, has challenges beyond that of drug or device-based treatments and we found that attempting to synthesise the results of trials of complex interventions like these presents particular difficulties compared to the synthesis of trials of simple interventions. We attempted to divide the different interventions into three disease management models proposed by Reigel (Riegel 2001): multidisciplinary; case management; and clinic. We recognise that there may be some overlap between these models and that some interventions are difficult to classify. Our understanding of the nature of the interventions was limited to published accounts. Most of the studies concern case management type interventions, although the content, intensity and duration of these interventions varied considerably. Only two studies have examined clinic interventions and there is only one RCT of a multidisciplinary intervention as defined by Riegel.
Although nine of the 16 studies appeared to be of at least moderate quality only seven studies definitely practiced allocation concealment and the outcome assessor was known to have been masked in only five studies. Only two studies mentioned how deaths and readmissions associated with heart failure were determined and none reported how this assessment was validated. The studies reported a wide variety of different outcomes and where only a few of the studies report a particular outcome there is the possibility of publication bias. It should also be noted that all but one of the studies were conducted in a single centre and all involved a selected population and it is not clear whether the benefits seen in these trials can be extrapolated to the wider population of patients with heart failure in whom co-morbidity is common (Havranek 2002).
Case Management interventions
All but one of these interventions involved telephone follow up from a respiratory nurse to the patient at home and many had a major educational component but the interventions did vary in their other components and their duration. The proportion of patients admitted with CHF who were eligible for the studies varied between 32% and 77% and the proportion of eligible patients who participated varied between 33 and 80%. There is some evidence from pooling the data in the highest quality studies that all cause mortality may be reduced with case management. No information was available on whether or not deaths associated with heart failure are influenced by case mmanagement Survival at six months follow up without death or unplanned readmission to hospital was reported in only one study but was significantly greater in the case managed group. From the studies that have been reported it is not clear whether survival without readmission to hospital for heart failure is influenced by case management at three and six months follow up, but two moderate quality case management studies reported very similar hazard ratios in favour case management at twelve months follow up. To date there is little evidence that all cause readmissions are significantly reduced by case management. There is however, some evidence from pooling the data that readmissions for heart failure may be reduced by case management, however because of the reservations described earlier this conclusion is tentative. There is little evidence that case management reduced the days spent in hospital during readmissions for any cause, but evidence from one study that days spent in unplanned readmissions may be reduced and evidence from two other studies that days spent in readmissions associated with heart failure or other cardiovascular problems may be reduced. There is also evidence that health related quality of life, as measured by the Minnesota Living with Heart Failure Questionnaire (MLHFQ) may be increased in patients receiving case management. The cost analyses are difficult to interpret and the data are sparse but suggest that case management interventions might be associated with cost reductions.
Clinic interventions
From the very scant evidence available, two studies both of which are likely to have lacked sufficient statistical power, there is almost no evidence of any benefit from clinic interventions. One of the two studies suggests that clinic based interventions are not feasible for elderly patients with a history of hospital admission for heart failure.
Multidisciplinary interventions
There is evidence from only one study on multidisciplinary type interventions. This suggests that a multidisciplinary type intervention may not improve event free survival, that is survival without admission to hospital for any reason, in the short term. This was the study’s primary endpoint and it appears to have had ssufficientstatistical power to examine this outcome. However the same study suggests that heart failure related re-admissions may be reduced in the short term (three months), but not the longer term (nine months), and hospital bed days may be reduced in the short term. It is not clear how generalisable these results might be - only 31% of the eligible patients were included in the study.
Day hospital Based Programme
The single study of a day hospital based heart failure programme directed at a very particular patient population (relatively young, male and many awaiting heart transplantation) showed a reduction in deaths from cardiac causes and hospital readmissions in the group receiving the intervention. A cost utility analysis suggested that over US$1000 would be saved with every quality adjusted life year (QALY) gained with the intervention, however the quality of this study is unclear and its results may not be generalisable.
AUTHORS’ CONCLUSIONS
Implications for practice
The data abstracted from this review form an insufficient basis for the formulation of firm recommendations for practice. Common components of most of the different disease management models appear to be the involvement of a specialist nurse, telephone follow up and patient education. Of the models examined most evidence concerns case management type interventions. There is some evidence that case management interventions may confer benefit in terms of overall survival and a tentative suggestion that they might be associated with a reduction in hospital readmissions for heart failure. Individual studies of case management interventions have shown some long term benefits in terms of unplanned hospital re-admissions or heart-failure related readmissions in single centre trials on selected study populations. There is also evidence that some case management interventions may be aassociatedwith improvements in health related quality of life. A single RCT of a multidisciplinary intervention showed evidence of benefits in terms of reduced heart-failure related re-admissions in the short term. There is at present insufficient evidence to support clinic based interventions and evidence from one feasibility study that they may not be feasible for heart failure patients.
Implications for research
Future studies of adequate sample size should include:
-
(1)
Multi centre RCTs looking at implementation of well-defined case management interventions or multidisciplinary interventions on study populations that are typical of patients admitted with CHF and which do not automatically exclude those patients living in residential care;
-
(2)
Comparisons between different interventions, particularly comparisons of interventions which have short duration (usually around discharge) and those which have a much longer duration;
-
(3)
The effect of interventions on patients’ and carers’ quality of life and their satisfaction with the interventions;
-
(4)
The cost effectiveness and cost-utility of interventions;
-
(5)
An examination of the core elements of these types of interventions.
There is a need establish sensitive and meaningful outcomes for these sort of disease management programmes.
PLAIN LANGUAGE SUMMARY.
Intense monitoring of patients with chronic heart failure following discharge from hospital - more studies needed
Patients with chronic heart failure (CHF) are often admitted to hospital as an emergency. The authors looked at 16 clinical trials that tested different ways of organising the care of CHF patients after they leave hospital. Only one of these trials was determined to be of high quality. There was some weak evidence that the intense monitoring of patients following discharge from hospital might improve survival and reduce the number of hospital readmissions. This type of care usually involved home visits and follow up telephone calls from specialist nurses. More research is needed.
ACKNOWLEDGEMENTS
We would like to than the following authors and researchers for their assistance:
Avitall B, Doughty RN, Ekman I (Ekman 1998), Hardman S, Harrison MB (Harrison 2002), Jaarsma T (Jaarsma 2000), Kasper EK (Kasper 2002), Krumholz HM (Krumholz 2002), Laramee AS (Laramee 2003), Massie B, McDonald K (McDonald 2002), Moser D, Rainville EC (Rainville 1999), Rich MW (Rich 1993; Rich 1995), Thompson DR
We are also grateful to Library Services at Queen Mary, University of London and to the following individuals: D Ashby, statistician; F Benato (assistance with reports in Spanish and Italian); A Besson, librarian; F Cason (assistance with reports in German); S Eldridge, statistician; A Spenser, economist and S Das; J Formby; E Hallgarten; A Langdon for assistance with searches or administrative support.
SOURCES OF SUPPORT
Internal sources
No sources of support supplied
External sources
ELENOR (East London and Essex Network of Researchers), UK.
DoH (Department of Health) Public Health Career Scientist Award, UK.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | RCT, single centre Recruiting: March 1997 to November 1998. Duration of follow up: 12 months (mean follow up) |
|
| Participants | Country: Scotland Participants: 81 patients (41 males, 51%) in comparison group, 84 (54 males, 64%) in intervention group Actual age of study subjects: usual care mean 75.6 years (SD 7.9), intervention 74.4 years (SD 8.6). Male sex: 58% Ethnicity: not given Actual severity of heart failure in study subjects at recruitment: NHYA class, n,: control group II 16 (20%), III 33 (42%), IV 30 (35%), intervention group II 19 (23%), III 28 (34%), IV 36 (43%) LVEF: not given Study inclusion criteria: Patients admitted as an emergency to the acute medical admissions unit at one hospital with HF due to LV systolic dysfunction Study exclusion criteria:
|
|
| Interventions | Duration of intervention: up to 12 months Intervention Group: “Specialist nurse intervention” During index hospitalisation: Patients were seen by a HF nurse prior to discharge. After discharge: Home visit by HF nurse and within 48 hours of discharge Subsequent visits by HF nurse at 1, 3, and 6 weeks and at 3, 6, 9 and 12 months. Scheduled phone calls at 2 weeks and at 1, 2,4,5,7,8,10 and 11 months after discharge. Patients and their families encouraged to contact nurses with problems or questions by phone during office hours (answering machine where they could leave messages after hours). Additional unscheduled home visits and telephone contacts as required Home visits covered: Patient education about HF and its Rx, self-monitoring and management (especially the early detection and treatment of decompensation). Patients were given a booklet about HF which included a list of their drugs, contact details for HF nurses, blood test results and clinic appointment times. The trained HF nurses used written drug protocols and aimed to optimise patient treatment (drugs, exercise and diet) and HF nurses also provided psychological support to the patient. HF nurses liaised with the cardiology team and other health care and social workers as required Comparison Group: usual care “Patients in the usual care group were managed as usual by the admitting physician and, subsequently, general practitioner. They were not seen by the specialist nurses after discharge.” |
|
| Outcomes | Primary endpoints: Unplanned re-admissions within 90 days of discharge. Total number of days hospitalised during follow up. Also looked at: Re-admission rates in the moderate risk subgroup compared to the high risk sub group Analysis done on intention to treat basis?: yes* |
|
| Notes | Data source: published data only Generalisability: 801 patients thought to have heart failure on admission were screened; 361 (45%) were eligible for the study and survived to have echocardiography; 12 (3%) refused consent; 184 (51% of 361) did not have LV systolic dysfunction; and 165 (46%, 21% of those screened) of these were randomised Consort flow chart: supplied. Rationale for sample size: given. Other points: Reasons for exclusions: proportions of patients with different reasons for exclusion not given Data on hospital admissions and deaths obtained both from the hospital records department and from the information and the statistics division of the Scottish NHS (admissions) and the Registrar General’s Office, Scotland (deaths) Generation of randomisation sequence and allocation concealment: “Study nurses phoned the Robertson Centre for Biostatistics and the patient was allocated to one or other randomisation group from a randomisation list.” Risk of care giver performance bias: possible, since HF nurses did not see control patients but hospital cardiology team may have been aware of randomisation group of patients. Risk of attrition bias: low. Risk of detection bias: low, “all hospital admissions were adjudicated blind to treatment” by a masked endpoint committee |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | RCT, single centre Recruitment: January 1999 to January 2000. Duration of follow up: mean follow up of 12 months |
|
| Participants | Country: Italy Participants: 122 patients (102 males, 84%) in comparison group, 112 (94 males, 84%) in intervention group Actual age of study subjects: mean age 56 years (SD 10) Male sex: 84% Ethnicity: not given. Actual severity of heart failure in study subjects at baseline: NYHA class I-II/III-IV: 158/81 (68% I-II) LVEF: 29% (SD 7) Study inclusion criteria:
Study exclusion criteria: None given. |
|
| Interventions | Duration of intervention: not clear. Intervention Group: Comprehensive Heart Failure Outpatient Management Program delivered by the day hospital During index hospitalisation: cardiac prognostic stratification and prescription of individual tailored therapy following guidelines and evidence After discharge: Attendance at day hospital staffed by a multidisciplinary team (cardiologist, nurse, physiotherapist, dietician, psychologist and social assistant). Patient access to the day hospital ’modulated according to demands of care process’. Care plan developed for each patient. Tailored interventions covering: cardiovascular risk stratification; tailored therapy; tailored physical training; counselling; checking clinical stability; correction of risk factors for haemodynamic instability; and health care education. Patients who deteriorate re-entered the day hospital through an open-access programme Day hospital also offered: intravenous therapy; laboratory examinations; and therapeutic changes as required The education given covered: knowledge about CHF and drug treatments and self management including daily weights, fluid restriction and nutrition Comparison Group: usual care During admission: cardiac prognostic stratification and prescription of individual tailored therapy following guidelines and evidence After discharge: ’The patient returned to the community and was followed up by a primary care physician with the support of a cardiologist’ |
|
| Outcomes | Primary outcomes: Readmissions because of haemodynamic instability. Deaths from cardiac causes. Cardiac mortality and urgent heart transplant Secondary outcomes: ‘Tailored therapy management’ QOL NYHA functional class Also looked at: Cost utility of the two strategies. Analysis done on intention to treat basis? Not clear |
|
| Notes | Data source: published data only Generalisability: 234 patients admitted the HFU with a diagnosis of CHF; 234 randomised (100%) Consort flow chart: not supplied Rationale for sample size: not given Other points: No patients excluded from study. Generation of randomisation sequence and allocation concealment: no information supplied Risk of care giver performance bias: unclear. Risk of attrition bias: unclear Risk of detection bias: likely because after 12 months all patients were re-evaluated in the Heart Failure Unit and the Day Hospital is part of this unit |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | RCT, single centre Recruitment: December 1991 to October 1993. Duration of follow up: 12 months. |
|
| Participants | Country: Sweden Participants: 110 patients (57 males, 52%) in comparison group, 80 (44 males, 55%) in intervention group. Actual age of study subjects: mean 75.6 years (SD 5.3) Male sex: 53% Ethnicity: not given Actual severity of heart failure in study subjects at baseline: NYHA class, mean: controls 2.6 (SD 0.7), intervention group 2.6 (SD 0.7) LVEF: control group mean 35.7% (SD 12.3), intervention group 31.6% (SD 8.4). (75% LVEF <40%) Study inclusion criteria:
Study exclusion criteria:
|
|
| Interventions | Duration of intervention: 12 months Intervention Group: “Management programme for heart failure” During index hospitalisation: Patients received an education programme from HF nurse consisting of two 30 minute visits After discharge: Two weeks after discharge patients and their families were invited to a one hour group education session led by the HF nurse which included an oral presentation by the nurse, and educational video and a question and answer session. Patients were also offered a seven day medication dispenser if deemed appropriate. Patients were followed up at a nurse directed o/p clinic and there was a single prescheduled visit by the nurse at 8 months after discharge. The HF nurse was available for phone contact during office hours. Patients encouraged to contact the study nurse at their discretion, if unsure, if diuretic adjustments did not ameliorate symptoms in 2-3 days, or if there were “profound changes in self management variables”. Patients were offered cardiology outpatient visits one and four months after discharge The inpatient and outpatient education programme covered: HF pathophysiology, pharmacological and non-pharmacological treatment. Patients were also given guidelines for self-management of diuretics in the event of fluid overload or fluid depletion. Patients were given a “heart failure diary” containing information on HF, list of HF medications, names and contact phone numbers for the HF clinic and in which to regularly record bodyweight, ankle circumference and HF symptoms Comparison Group: usual care These patients were “followed up at the outpatient clinic in the department of cardiology by either cardiologists in private practice or by primary care physicians as considered appropriate by the discharging consultant.” |
|
| Outcomes | Primary endpoint: Not specified, abstract states that main outcome measures were: time to re-admission, days in hospital and health care costs during one year Other endpoints: Quality of life using The Quality of Life in Heart Failure Questionnaire, Nottingham Health Profile and patients’ global self assessment (all self-administered) Also looked at: Deaths at 90 days Event free (i.e. death or re-admission) survival at 90 days Analysis done on intention to treat basis?: unclear |
|
| Notes | Data source: published data only Generalisability: no information supplied on number of patients screened for entry to the study or on the number of patients excluded. 206 eligible patients were randomised before consenting, 16 patients (8%) randomised to the intervention group withheld their consent, no patient randomised to the control group withheld consent Consort flow chart: not supplied Rationale for sample size: not given Other points: Reasons for exclusions: proportions of patients with different reasons for exclusion not given Generation of randomisation sequence: computer generated random allocation. Allocation concealment: “Patients were invited to participate and informed consent was given on the basis of information relevant to the allocated study group. This procedure avoided bias arising from control patients being informed of the intervention strategy.” Risk of care giver performance bias: possible that some of the control patients were also seen by cardiologists involved in the study. Risk of attrition bias: low “all patients were accounted for”. Risk of detection bias: possible, not clear who collected data on patients and not clear if this data collection was masked |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Cluster RCT, GP as the unit of randomisation (but see note), single centre. Recruitment: during 1997 and 1998. Duration of follow up: 12 months. |
|
| Participants | Country: New Zealand Participants: 97 patients (54 males, 56%) in comparison group, 100 (64 males, 64%) in intervention group. Actual age of study subjects: mean 73 years (SD 10.8, range 34 to 92 years). Male sex: 60% Ethnicity: ‘NZ European’ 79% Severity of heart failure in study subjects: (At index admission) NYHA class, n (%): controls II 24 (25%), III 73 (75%), intervention group II 24 (24%), III 76 (76%). (At baseline) LVEF: control group mean 33.8% (SD 12.7), intervention group 30.6% (SD 12.7) Study inclusion criteria: Patients admitted to general medical wards with a primary diagnosis of heart failure Study exclusion criteria:
|
|
| Interventions | Duration of intervention: 12 months Intervention Group: ‘integrated heart failure management programme’ After discharge: Outpatient review at heart failure clinic within 2/52 of discharge from hospital: clinical status reviewed, pharmacological treatment based on evidence based guidelines, one-to-one education with study nurse, education booklet provided. Patient diary for daily weights, Rx record & clinical notes provided. Detailed letter faxed to GP and follow up phone call to GP. GPs encouraged to discuss management with clinic team. Follow up plan aiming at 6 weekly visits alternating between GP and HF clinic. Group education sessions for patients run by cardiologist and study nurse: two sessions offered within 6 weeks of discharge and one at 6 months post d/c. Telephone access to study team for GPs or patients during office hours Group education sessions covered: education about disease; monitoring daily body weight and action plans for weight changes; medication; exercise; diet. Comparison Group: usual care |
|
| Outcomes | Primary endpoints: Time to first event i.e. death or hospital re-admission. HRQL measured using Minnesota Living With Heart Failure Q at baseline and 12 months Other endpoints: All cause hospital re-admissions. Heart failure related hospital re-admissions. All cause hospital bed-days Also looked at: Medications at 12 months Analysis done on intention to treat basis?: yes |
|
| Notes | Data source: published data only Generalisability: does not report how many patients were screened for eligibility to study, nor how many of those deemed eligible agreed to participate Consort flow chart: not supplied Rationale for sample size: given but sample size calculation not verifiable from information given and no mention of adjustment of sample size calculation from cluster randomised design. (Study terminated early before sample size achieved.) Other points: Reasons for exclusions: proportions of patients with different reasons for exclusion not given. Randomisation: GPs were randomised before participant recruitment - possibility that team were aware of assignment of GP before recruitment of patient into study. Generation of randomisation sequence and allocation concealment: “General practitioners were randomly allocated using computer generated random numbers…after consent was obtained the patient was informed of their group allocation based on the randomisation of their current general practitioner.” Care giver performance bias: unclear; primary care giver performance bias unlikely because to avoid contamination of GPs a cluster RCT design was employed. However, not clear whether hospital staff managed both intervention and control patients. Risk of attrition bias: low Risk of detection bias: possible, no mention of blinding of those assessing endpoints |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | RCT, feasibility study, one centre Recruitment: November 1994 to January 1996. Duration of follow up: mean follow up time 5.0 months (SD 2.0) control group and 5.0 (2.3) in the intervention group |
|
| Participants | Country: Sweden Participants: 79 patients in comparison group, 79 in intervention group, males: females in each group not given. Actual age of study subjects: mean 80.3 years (SD 6.8) Male sex: 58% Ethnicity: not given Actual severity of heart failure in study subjects at recruitment: NYHA class: control group mean 3.2 (SD 0.5), intervention group 3.2 (SD 0.5) LVEF on 99 patients (63%): control group mean 38% (SD 25), intervention group 43% (SD 18) Study inclusion criteria:
Study exclusion criteria:
|
|
| Interventions | Duration of intervention: 6 months? Intervention Group: ‘Structured care programme based on Nurse-monitored outpatient clinic’ After discharge: Pt and carers offered visit to specialist nurse monitored HF clinic one week after discharge, clinic run in collaboration with the study doctors (who were responsible for pharmacological Rx). Main aim of programme was patient education about their treatment and the symptoms of clinical deterioration. Tailored care plan with individualised treatment goals for each patient. Primary care team continually informed about patient’s situation by HF clinic nurses. Patients had access to clinic nurses during business hours. In emergencies patients seen by clinic nurses and attending doctor Patients given notebook for daily weight monitoring, treatment and information about clinical deterioration. Clinic nurses made regular follow up telephone calls to patients, those not seen regularly in clinic were called monthly Comparison Group: usual care. In general this was GP follow up. |
|
| Outcomes | Main endpoints: Proportion of patients aged > 65 years who were eligible for the study. Proportion of patients in the intervention group who did not visit the HF nurses. NYHA functional class. Hospitalisations and hospital days during six month follow up. Deaths. Analysis done on intention to treat basis?: yes |
|
| Notes | Data source: published data and information from author* Generalisability: Of 1058 consecutively screened patients with a diagnosis of heart failure and cardiomyopathy and aged 65 years or older, only 160 (17%) met criteria for participating in the study (2 later found not to be eligible) and 22 (12%) of these refused to participate Consort flow chart: not supplied Rationale for sample size: not given, feasibility study Other points: Reasons for exclusions (sub study of 454 excluded patients): 27% had serious communication problems or were otherwise too disabled to attend the out patient clinic, 25% had Boston criteria score <8, 18% NYHA class <III, 8% nursing home care, 7% specialist care, 5% acute MI Generation of randomisation sequence: “randomly permuted blocks with a size of 20 obtained from tables of random numbers.”. Allocation concealment: “consecutively numbered, sealed envelopes containing group assignments”. The envelopes were generated by a doctor but allocated by a nurse Risk of care giver performance bias: high; 20 care likely because the three specialist nurses who staffed the HF clinic also staffed the inpatient ward, 10 care both control and intervention patients’ general practitioners were aware their patients were in the study* and they must have known the allocation group of their patients. Risk of attrition bias: low Risk of detection bias: high, those collecting endpoint data were not masked to patients’ allocation status* |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | RCT, single centre Study recruitment: June 1996 to January 1998. Duration of follow up: 12 weeks. |
|
| Participants | Country: Canada Participants: 100 patients (56 males, 56%) in comparison group, 92 (49 males, 53%) in intervention group. Actual age of study subjects: mean age 76 years, median age 77 years (range 33-93 years). Male sex: 55% Ethnicity: not given. Severity of heart failure in study subjects: NYHA class, n (%) at baseline: controls: I 2 (2%), II 20 (20%), III 69 (69%), IV 8 (8%); intervention group: I 0, II 21 (23%), III 60 (65%), IV 11 (12%) LVEF: not given Study inclusion criteria:
Study exclusion criteria:
|
|
| Interventions | Duration of intervention: 2 weeks following discharge. Intervention Group: Transitional care (TC) Before discharge: Standard discharge planning and care (see below). Comprehensive, evidence based education programme for heart failure self-management (PCCHF). A nursing transfer letter to the home care nurse detailing clinical status and self-management needs After discharge: Phone call from hospital nurse to patient within 24 hours of discharge. Minimum of two community nurse visits within two weeks of discharge Content of PCCHF: Patient workbook covering: the disease, self-monitoring, management of medication, diet, exercise, stress, support systems and community resources. Allowed tailoring for individual needs. Also contained an education plan and served as a patient held documentation tool Comparison Group: usual care discharge planning and ‘optimal’ usual post discharge care. Before discharge: Ideally a multidisciplinary discharge plan within 24 hours of admission and weekly discharge planning meetings. Regional home care co-coordinator consults with hospital team as required and may meet patients and their families. Immediately before discharge physician completes referral form for home care and necessary services and supplies are communicated with the home nursing agency After discharge: Number of home visits scheduled to match those received by TC group |
|
| Outcomes | Primary outcome: HRQL measured by the MLHFQ 6 and 12 weeks post discharge. Secondary outcomes: QOL measured with SF-36. Number of all-cause emergency room visits. Number of all-cause hospital re-admissions. Analysis done on intention to treat basis?: no “Eleven individuals were readmitted to hospital during the intervention period. Timing for outcome measures then began on second discharge and followed for 3 months. ” Also, eight patients who dropped out after randomisation were not included in the study |
|
| Notes | Data source: published data and information from author*. Generalisability: 483 patients thought to have heart failure admitted; 212 (44%) were eligible for the study; 12 (6%) refused consent. After randomisation 8 more patients (5 TC and 3 usual care) did not enter the study: four died or became too ill; two refused home care; one changed diagnosis and one was discharged to long term care. 192 patients (40% of admissions) were entered into the study, of these only 157 (82% of those considered entered into study, 78% of those randomised) were followed up Consort flow chart: not supplied Rationale for sample size: given Other points: Reasons for exclusions (271 patients): coming from/ being discharge to long term care (38%); living outside the catchment area (23%); too ill or deceased shortly after admission (15%); first language not French or English (12%); discharged with in 24 hours (6%); diagnosis changed (3%); other (3%) Not clear whether, or how many of, the eleven readmitted and re-entered patients were in intervention or control groups. Generation of randomisation sequence: computer generated schedule. Allocation concealment: “pre-packaged, consecutively numbered, sealed opaque envelopes containing the group allocation were prepared for each nursing unit and administered from the research office. Neither the patients nor the members of the study team were aware of treatment assignment until after randomisation.” Care giver performance bias comment: bias possible since hospital nurses provided both experimental and control interventions and all care givers in the community were informed that patients were in the intervention or control groups after randomisation*. Risk of attrition bias: likely. Only 157 patients (82% of those considered entered into study, 78% of those randomised) were followed up. 23 UC patients and 12 TC patients not followed up, reasons: died or too ill (11 UC, 9TC); withdrew (7 UC, 1 TC); lost to follow up (5 UC, 2 TC). Risk of detection bias: low, outcome assessors masked*. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | RCT Recruitment: May 1994 to March 1997. Duration of follow up: 9 months. |
|
| Participants | Country: the Netherlands Participants (patients enrolled and surviving to discharge): 95 patients in comparison group, 84 in intervention group* Actual age of study subjects: not given for original group, those who remained at 9 months were mean age 72 years (SD 9) at baseline. Male sex: of those who remained at 9 months, 60% Ethnicity: not given Actual severity of heart failure in study subjects at recruitment: not known Study inclusion criteria:
Study exclusion criteria:
|
|
| Interventions | Duration of intervention: up to 10 days after discharge from index admission, on average one week* Intervention Group: ‘Supportive educational intervention’ During index admission: Intensive education by study nurse using standard nursing care plan After discharge: Study nurse phoned patient within one week of discharge to assess potential problems and made appointment for home visit. Home visit on average one week after discharge*. At home visit education continued If required, study nurse wrote to patient’s home care nurse about patient’s specific needs. Between discharge and home visit patient could contact study nurse if they encountered problems. After home visit patient encouraged to contact their cardiologist, GP or emergency heart centre with any problems. Educational component covered: symptoms of worsening failure, sodium restriction, fluid balance and compliance and individuals’ problems, and included education and support to patients’ family Comparison Group: usual care. “A nurse or physician, depending on his or her individual insight into the patients’ questions, provided these patients with education about medication and lifestyle”. Usual care patients did not receive structured education |
|
| Outcomes | Primary endpoints: none specified Measures of QOL: Heart Failure Functional Status Inventory (to assess functional capabilities at baseline, 3 and 9 months). Symptom occurrence (at baseline, 1, 3 and 9 months), severity and distress questionnaire, designed for this study (at 3 and 9 months). Psychosocial Adjustment to Illness Scale (at baseline, 3 and 9 months). Cantril’s Ladder of Life (to measure overall well being at baseline, 1, 3 and 9 months) Measures of self-agency and self-care behaviour: The patients’ ability to care for themselves using the Appraisal of Self-care Agency Scale (ASE) (at baseline, 3 and 9 months). The patients’ self care behaviour using a Heart Failure Self-care Behaviour Scale, designed for this study (at baseline, 1, 3 and 9 months) Healthcare resource use: Patients’ report of number and reason for contact with GP, cardiologist, medical specialists, physical therapists, social care providers and alternative health specialists. Hospital readmissions and out patient visits from hospital database. Reasons for readmission form patient charts. Also reported: Deaths at 9 months. Analysis done on intention to treat basis? No, some adjustments for attrition made |
|
| Notes | Data source: published data and author contacted for clarification (indicated by *) Generalisability: * Of 828 admissions to the ward with heart failure; 184 (22%) were re-admissions; 66 patients were not screened and 14 died during screening. 564 (68%) patients met inclusion criteria; 352 of these (62%) were excluded; 40 (7% of 564) did not give informed consent, 186 (33% of 564, 22% of the 828 admissions) were randomised of whom 7 died before discharge Consort flow chart: not supplied Rationale for sample size: Power was calculated for psychosocial adjustment to illness as measured by the PAIS and based on data from a HF sample in the USA. It was calculated that two groups of 58 patients would be required to show an 8 point difference in the PAIS with an a of 0.005 and a ß of 0.9* Other points: Reason for exclusions (352 patients): *history < 3 months 171 (49%); psychiatric disturbance, dementia or cancer 31 (9%); NYHA class <III or cardiac intervention 22 (6%); Boston score <6 12 (3%); age <50 years 12 (3%); discharged to a nursing home 9 (3%); language 5 (1%); >1 exclusion criteria 76 (22%) *The symptom occurrence, severity and distress questionnaire was designed for this study but was derived from pre-existing literature. The data were based on patient self report. Experts in the field assessed content validity, but otherwise the validity and reliability of the questionnaire had not been formally examined The Heart failure Self-care Behavior Scale was designed for this study and validated by a panel of experts Generation of randomisation sequence and allocation concealment: “By drawing from an envelope patients were randomly assigned to receive either care-as-usual or the supportive-education intervention”. Risk of care giver performance bias: low; “Health care personnel (cardiologists or staff) involved in the care for the patients did not know if the patient was in the intervention or control group.” Risk of attrition bias: possible, 186 patients enrolled in to the study and 132 (71%) remained at 9 months. 58/84 (69%) remained in the intervention group whilst 74/95 (78%) in the control group, NS, there was a trend towards more patients with NYHA IV dropping out. Analyses on self-care abilities and behaviour were adjusted in an attempt to compensate for the influence of attrition - this adjustment assumed that those who dropped out did not improve their self-care and self-agency from baseline this assumption may not have adequately adjusted for attrition. Risk of detection bias: high; the two study nurses who delivered the intervention were also involved in the study as data collectors and were aware of the allocation status of the patients |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | RCT, two centres Recruitment: December 1996 to December 1998. Duration of follow up: Six months from recruitment (plus additional three months) |
|
| Participants | Country: USA Participants: 102 patients (66 males, 65%) in intervention group, 98 (55 males, 56%) in comparison group. Actual age of study subjects at recruitment: median 63.5 years (range 25-88 years) Male sex: 61% Ethnicity: ‘white’ 64% Actual severity of heart failure in study subjects at baseline: NYHA class, n (%): controls II 33 (34%), III 60 (61%), intervention group II 38 (37%), III 57 (56%). LVEF: control group mean 27.5% (SD 13.9, range 5-60), intervention group 27.1% (SD 13.8, range 10-70) Study inclusion criteria:
|
|
| Interventions | Duration of intervention: 6 months. Intervention Group: ’multidisciplinary program’ During index hospitalisation: CHF cardiologist designed an individualised treatment plan for each patient before randomisation which included medication, diet and exercise management After discharge: ‘Telephone nurse co-coordinator’ phoned patients within 72 hours of discharge and then weekly for 1st month, bi-weekly in 2nd month and then monthly. (Content of phone calls: set script with problems pursued as clinically indicated . No medication adjustments over phone.) Monthly follow up with CHF nurses (usually in CHF clinic). ‘Primary care physicians’ (66% internal medicine physicians, 29% cardiologists) received regular updates from CHF nurses and were notified of abnormal lab results. All intervention patients received: pill sorter, list correct medications, list of dietary and exercise recommendations, 24 hour telephone contact number and patient educational material. If required and financial resources limited patients also received: 3g sodium ‘Meals on Wheels’ diet, weigh scale, medications, transport to the clinic and a phone. CHF cardiologist saw patients at 6 months. Content of CHF nurse follow up: aimed to implement the treatment plan designed by CHF cardiologist by using a pre-specified 55 page algorithm (also designed by the CHF cardiologists) which included initiation and titration of drugs, a low sodium diet and exercise recommendations Comparison group: Usual care. This was care by the patients’ primary physicians (73% internal medicine physicians, 26% cardiologists). CHF cardiologist designed treatment plan for each patient “documented in patient’s chart without further intervention” |
|
| Outcomes | Primary endpoint: Total number of CHF hospital admissions plus all cause deaths (i.e. composite endpoint) Secondary outcomes: Death. CHF hospital admissions. All cause hospital admissions. Change in HRQOL (MLHFQ). Change in activity status (Duke Activity Status Index). Process indicators including: proportion of patients with systolic dysfunction receiving ACEI according to published guidelines or appropriate alternative treatment if intolerant of ACEI; percentage patients euvolemic according to defined goal weight; compliance with dietary guidelines using locally developed sodium score and cost data |
|
| Notes | Data source: published data and information supplied by author* Generalisability: 1,452 patients with heart failure were screened, (screened patients were not consecutive admissions*); 976 (67% of those screened) met inclusion criteria of whom 686 (70%) had one or more exclusion criteria; of the remaining 290 eligible subjects 90* (31%) refused to participate Consort flow chart: not supplied Rationale for sample size: supplied Other points: Reason for exclusions (based on 776 patients, i.e. 686 excluded patients and 90 patients who refused): participating in another research protocol 15.5%; renal dysfunction 14.3%; dementia or substance abuse 10.7%; planned cardiac revascularisation or heart transplant 10.2%; cardiac exclusions such as hypertrophic cardiomyopathy, restrictive cardiomyopathy, amyloidosis, valvular heart disease 10.2%; living outside catchment area 7%; discharged to nursing home or on intravenous inotrope 5.1%; non-cardiac disorder likely to cause repeated hospital admission 12.8%; primary care physician declined to participate 2.3% Intervention and control groups appear similar at baseline but some inclusion criteria data not presented Three patients required heart transplantation (two during study and one immediately after) despite the exclusion criterion of heart transplantation likely to occur within six months Funding: Partial funding was provided by CardioContinuum, Inc. This company, the University and its Division of Cardiology are entitled to royalty on the use of the CHF management programme described in the study. The University also owns CardioContinuum stock. None of the investigators has personal royalty interests, stock or consulting arrangements with CardioContinuum, except for one who was once a CardioContinuum employee. Generation of randomisation sequence and allocation concealment: “The coordinating centre made treatment assignments by using an automated telephone response system…Random number schedules were prepared before initiation of patient recruitment ands were unknown to the clinical investigators.” Comment on care giver performance bias: physicians providing usual care were aware of study and knew that their patient had not been allocated to the intervention so possible Hawthorn effect on care received by the usual care group. *Also 10 % of these physicians had other patients allocated to the intervention arm which may also have influenced their usual care. The effect of both of these influences would be to underestimate the effect of the intervention. Risk of attrition bias: low. Risk of detection bias: low for main outcomes. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | RCT, single centre Recruitment period: October 1997 to September 1998. Duration of follow up: one year. |
|
| Participants | Country: USA Participants: 44 patients (29 males, 66%) in comparison group, 44 (21 males, 48%) in intervention group. Actual age of study subjects: median age 74 years, controls mean age 71.6 (SD 10.3), intervention 75.9 (SD 8.7) Males: 57% Ethnic group: ‘74% caucasians’ Actual severity of heart failure in study subjects at recruitment: Mean ejection fraction: control group 37% (SD 16), intervention group 38% (SD 17). NYHA: not given Study inclusion criteria:
Study exclusion criteria:
|
|
| Interventions | Duration of intervention: one year Intervention Group: ‘Education and Support’ After discharge: Initial hour long face to face consultation with experienced cardiac nurse within two weeks of discharge using a teaching booklet (45% of these consultations took place in patient’s home, remainder in hospital clinic). Following this weekly telephone contact for four weeks, bi-weekly for eight weeks then monthly until one year Initial consultation covered five sequential care domains for chronic illness including: patient knowledge of illness; the relation between medication and illness; the relation between health behaviours and illness; knowledge of early signs and symptoms of decompensation, and where and when to obtain assistance. Follow up phone calls reinforced the five care domains but did not modify current regimens or provide recommendations about treatment. However the nurse could recommend that the patient consulted his/her physician when the patient’s condition deteriorated sharply or when the patient had problems, in order to help patients to understand when and how to seek and access care Comparison Group: usual care. All usual care treatments and services ordered by their physicians |
|
| Outcomes | Primary endpoint: Re-admission or death. Secondary endpoints: All cause admissions. HF related or other CVD related re-admissions. Cumulative number of days in hospital. Cost of readmission. Analysis done on intention to treat basis? Yes |
|
| Notes | Data source: published data and information from author*. Generalisability: 390 consecutive admissions who met clinical criteria for HF screened, 142(36%) eligible and a further 34 eligible but not enrolled, 20 (5%) patient, physician or family refusal to participate, 88 (23% of those screened) enrolled in the study Consort flow chart: not supplied Rationale for sample size: provided, they expected a 40% reduction in readmission or death from 75%. The number required to show this not stated in text Other points: Reasons for exclusions (248 patients): admitted from nursing home 19%; transfer from another acute facility (18%); conditions interfering with interview 18%, elective admission 12%, already enrolled in study 12%; high output state 6%; other terminal disease 4%; terminal or skilled nursing care 4%; enrolled in other studies 3%; no signs/symptoms of HF 3%; other impairing conditions 2%, cardiomyopathy 1 %, age <50 1%, followed by another facility 1%. A further 34 were eligible but not enrolled because of no interview because of death, discharge or other medical reasons 65%, no telephone or residing in another state 35% Generation of randomisation sequence: “computer generated”* Allocation concealment: “It was not blinded”* Comment on risk of care giver performance bias: low, care givers were not informed of patient’s involvement in the study by the researchers*. Risk of attrition bias: Risk of detection bias: low, record examinations to confirm events and classify cause done by a clinician masked to patient’s intervention allocation |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | No | C - Inadequate |
| Methods | RCT, one centre Recruitment period: July 1999 to April 2001 Duration of follow up: 90 days |
|
| Participants | Country: USA Participants: 146 patients (74 males, 50 %) in comparison group, 141 (82 males, 58%) in intervention group. Actual age of study subjects: mean 70.7 years (SD 11.8) Male sex: 54% Ethnicity: not given Severity of heart failure in study subjects: (At enrolment) NYHA class, n (%): controls I 35 (26%), II 47 (36%), III 46 (35%), IV 4 (3%) intervention group I 10 (7%), II 76 (55%), III 50 (36%), IV 3 (2%). (At enrolment) LV dysfunction: control group normal to mild 29 (21%), moderate 26 (19%), moderate-severe 22 (16%), severe 58 (43%), intervention group normal to mild 27 (20%), moderate 15 (11%), moderate-severe 20 (15%), severe 73 (54%) Study inclusion criteria:
Study exclusion criteria:
|
|
| Interventions | Duration of intervention: 12 weeks Intervention Group: ‘Case management’ Before discharge: Education by study nurse case manager. Early discharge planning and co-ordination of care by case manger including: arranging for consultations are required with physical therapy and occupational therapy; facilitating communication between hospital team and patient and family; submitting progress reports to primary care physicians; involving patient and family in developing care plan; collaborating with home health agencies and providing information and emotional support to patient and family. Patients received a educational booklet; weight logs; medication lists and a guide for measuring sodium intake also weigh scales and pill boxes as required After discharge: Letter to patient’s doctors informing them of participation in the study and outlining the case management programme. Scheduled telephone calls by case manager to patient at home at 1-3 days after discharge, then weekly for first month followed by fortnightly for next two months. Patients were also able to contact the case manager during weekday office hours. At six weeks post discharge the patient’s responsible physicians were contacted if their patients were not on medications or doses as outlined in the treatment plan Telephone calls covered symptoms and adherence to all aspects of treatment plan, resources available, next appointment time with primary care provider, reinforced education plan with patient and family and provided an opportunity for patients and family to ask questions. In the event of symptoms of worsening heart failure ‘appropriate triage’ was arranged and additional phone calls made to patients The education plan was consistent with education guidelines and covered: disease process of CHF; diet and fluid intake recommendations; medications and dosing plan; self-monitoring of signs and symptoms of CHF; activity recommendations; cardiac risk factor modification; prognosis and counselling Comparison Group: usual care Standard care ‘typical of a tertiary care hospital’ including opportunity for social services evaluation, dietician consultation etc and home care service on discharge. Post discharge care conducted by the patient’s own local physician |
|
| Outcomes | Primary endpoint: All cause readmissions during 90 days after discharge. Secondary endpoints: Adherence to the treatment plan. Patient satisfaction, dosages of : angiotensin-converting enzyme inhibitors; angiotensin recptor blockers; and beta blockers, overall cost of medical care Also looked at: Cause for readmission Length of stay Number of CHF readmissions Cumulative number of hospital days Number of days to first readmission Analysis done on intention to treat basis?: no*. |
|
| Notes | Data source: published data and information from author*. Generalisability: 589 patients thought to have heart failure admission were screened; 454 (77%) were eligible for the study; 74 (16%) refused consent; 13 (3%) patient’s physicians refused consent; 80 (18%) were not randomised for logistical reasons including patient discharged before consented; and 287 (63%, 49% of those screened) of these were randomised Consort flow chart: supplied Rationale for sample size: not given explicitly, but does say that they were seeking to show a 50% reduction in 90 day readmission rates Other points: Reasons for exclusions (135 patients): discharged to long term care 32%; planned cardiac surgery 31%; cognitive impairment 21%; expected survival <3 months 10%; haemodialysis 6% Adherence to treatment survey instrument not validated. Generation of randomisation sequence and allocation concealment: They used a block randomisation in order to balance out the workload of the case manager so she would not have a chance of getting more than five patients in a row. They used 10 envelopes: five for each group and let the patient choose an envelope to determine what group they were in. When 10 Patients had picked the 10 envelopes they started again with 10 envelopes. Patients were recruited in order of their admission date and time. “Hence this was not a blinded study for participants or researchers.”* Care giver performance bias comment: possible because hospital teams would know who was receiving intervention. Risk of attrition bias: low, although 19 patients (13%) not followed up in usual care group (9 lost to follow up; 9 withdrew consent; 1 ‘no longer met criteria’) compared to 6 (4%) lost to follow up in intervention group. Risk of detection bias: possible, clinical research co-coordinator enrolled patients in study out come data collected by clinical research co-coordinator (usual care group) and case manager (intervention group) |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | No | C - Inadequate |
| Methods | RCT, single centre Recruitment: November 1998 to April 2000. Duration of follow up: 12 weeks. |
|
| Participants | Country: Ireland Participants: 47 patients (33 males, 70%) in comparison group, 51 (32 males, 63%) in intervention group Actual age of study subjects: mean 70.8 years (SD 10.47). Male sex: 66%. Ethnicity: not given Actual severity of heart failure in study subjects at recruitment or at baseline: NYHA: not given. LVEF: control group mean 38% (SD 15), intervention group 36% (SD 12) Study inclusion criteria:
Study exclusion criteria: Heart failure in the presence of MI or unstable angina. Requiring immediate valve surgery. Patients “in which heart failure was not considered to be the primary problem”. “Illnesses which would compromise survival” over the next 12 weeks. Cognitive impairment. Resident in a nursing home. Significant hearing/visual impairment. Living abroad. Not English speaking. |
|
| Interventions | Duration of intervention: 12 weeks Intervention Group: ‘Multidisciplinary care’ During index admission: “Optimisation” of medical therapy and satisfying predefined clinical and therapeutic stability criteria before discharge Specialist HF nurse led patient education and dietician consultations on three or more occasions After discharge: Letter sent requesting primary physician to refer HF related issues to HF clinic or HF specialist nurse. HF nurse telephoned patient within 3 days of discharge and weekly thereafter. Telephone calls covered clinical status, problems and key education issues as required. Patients and carer or next of kin seen in HF clinic in 2nd and 6th week after discharge. At clinic visits clinical status checked and key educational issues covered. U&E were checked at clinic and if necessary clinic had option of using intravenous frusemide “to regain outpatient clinical stability”. Patients were also asked to contact the HF clinic if they noticed any clinical deterioration - diuretics were increased if weight increased >= 2kg. The decision to admit patients to inpatient care was governed by specific, pre-defined criteria. Review in HF clinic at 3 months. The educational intervention focussed on: Understanding disease and medication. Daily weight monitoring. Dietary salt restriction. Similar advice was given to patient’s carer or next of kin where applicable Optimisation of care whilst an inpatient involved echocardiography. Patients with ejection fraction <45% were prescribed diuretics, digoxin and prescribed ACEI at maximally tolerated does. Those with normal systolic function were managed as deemed appropriate by their cardiologist. Patients had to meet predefined criteria for clinical and therapeutic stability before they were discharged Comparison Group: “Optimisation” of medical therapy and satisfying predefined clinical and therapeutic stability criteria before discharge (see above). On discharge referred back to primary physician and usual care. “The decision to admit a patient was the responsibility of the physicians in charge of care and was not influenced by the persons involved in the study”. Review in HF clinic at 3 months. |
|
| Outcomes | Primary endpoint: Number of patients with death or re-admission for HF within 12 weeks (composite endpoint) Also looked at: Patient and carer knowledge of HF (20 question questionnaire) on discharge from index admission and at 30 days. Patient and carer knowledge of the importance of diet (10 questions) on discharge from index admission and at 30 days. An earlier paper (McDonald 2001) reports on deaths and re-admission to hospital for HF at 30 days in the first 70 Patients enrolled in the study Analyses done on an intention to treat basis?: yes. |
|
| Notes | Data source: published data and information supplied by author* Generalisability: Of 337 patients admitted via accident and emergency with a presumed diagnosis of HF, 214 had a primary diagnosis of HF, 7 (3%) died in hospital, 84 (39%) met other exclusion criteria, 123 (57%, 36% of 337) eligible and survived to d/c, 25 (12%) refused to participate Consort flow chart: provided. Rationale for sample size: not given Other points: Reasons for exclusions: proportions of patients with different reasons for exclusion not given. Questionnaires were generated by the study team and “were not pre-tested or validated in any way”. Not clear if consecutive patients were recruited. Generation of randomisation sequence and allocation concealment: “A randomisation list was generated … before [completion] of the study protocol. A randomisation number was allocated to 200 sequential study patient numbers. The allocation of an odd number designated the corresponding study patient number as routine care and vice versa. This randomisation list was retained off site …and was not accessible to the physicians involved in the study.” Following inclusion in the study the physician telephoned the administrator’s office and was informed of the allocation group of the patient.* Care giver performance bias possible because control group patients could be referred back to study team. Risk of attrition bias: low. Risk of detection bias: high, see comments on detection of primary endpoint in results table and in text |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | RCT, single centre Recruitment: July 1996 to June 1997 Duration of follow up:12 months |
|
| Participants | Country: USA Participants: 17 patients (9 53% males) in comparison group, 17 (8 males 47%) in intervention group, Actual age of study subjects: control group mean 72.8 years (SD 10.7), intervention group 66.9 (SD 8.7). Male sex: 50% Ethnicity: not given Actual severity of heart failure in study subjects at recruitment: NHYA class, n, (%): control group II 4 (24%), III 11 (65%), IV 2 (10%), intervention group II 1 (6%), III 12 (71%), IV 4 (24%). LVEF: not given. Study inclusion criteria:
Study exclusion criteria:
|
|
| Interventions | Duration of intervention: 90 days Intervention Group: ‘pharmacist intervention’ During index hospitalisation: “Routine care plus pharmacist and clinical nurse specialist identified patient issues which posed risk for rehospitalisation and determined corrective action.” Before discharge the pharmacist reviewed pathology and treatment of HF, weight monitoring and risk modifications with the patient or caregiver. Patient given information brochure, video, weight log and medication organiser. Pharmacist also recommended medication changes to physicians. After discharge: Pharmacist phoned within three days of discharge, and at 7, 30, and 90 days and 12 months to enquire about any re-admissions, respond to questions, reinforce information give before discharge. Pharmacist’s phone number provided to patients for further support Comparison Group: usual care Routine care and preparation for discharge including: written prescription, physician discharge instructions, nurse review of diet, treatment plans and medications; patients provided with computer generated drug information sheets. At 30, and 90 days and 12 months pharmacist contacted patients to ask about re-admissions |
|
| Outcomes | Primary endpoint: hospital re-admission for heart failure or death (composite endpoint) Analysis done on intention to treat basis: No* |
|
| Notes | Data source: published data and information from author* Generalisability: *Of 377 patients whose admission history included HF, 42 refused consent (11%) and 42 (11%) were eligible and provided informed consent (3 patients then became ineligible during index admission, 1 patient lost to follow up). Data on these four patients were excluded Consort flow chart: not supplied Rationale for sample size: not given Other points: Reasons for exclusion (288 patients): unstable coronary disease 78 (27%), pulmonary disease 37 (13%), cardiac arrhythmia 33 (11%), pneumonia 20 (7%). *Heart failure re-admissions were confirmed either by chart documentation or personal communication with the admitting physician. Generation of randomisation sequence: computer generated* Allocation concealment: “Information on patient randomisation was concealed from the patient and all care givers except for the pharmacists involved in the study”. It is not clear who was responsible for allocation Risk of care giver performance bias: low* Risk of attrition bias: low Risk of detection bias: high |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | RCT, single centre, pilot study Recruitment: April 1988 to March 1989. Duration of follow up: 90 days from hospital discharge. |
|
| Participants | Country: USA Participants: 35 patients (15 males, 43%) in comparison group, 63 (25 males, 40%) in intervention group, (2:1 assignment to intervention or usual care). Actual age of study subjects: mean age 79 years (SD 6). Male sex: 41%. Ethnicity: “white” 50% Actual severity of heart failure in study subjects at recruitment: NYHA class, mean: controls 3.0 (SD 1.0), intervention group 2.7 (SD 1.1) Study inclusion criteria:
Study exclusion criteria:
|
|
| Interventions | Duration of intervention: not clear, during index hospitalisation, plus one week after discharge, plus phone follow up Intervention Group: ‘Comprehensive multidisciplinary treatment strategy’ During index hospitalisation: Daily educational visits by study specialist nurse. Dietician visit with individualised 1.5-2.0 g sodium diet. Medication review by geriatric cardiologist with patient and/or caregivers. Drug regimen rationalised with aim of maximising compliance and minimising side effects and drug interactions. Following this study nurse taught patients about medications and dosing, medication cards and charts provided and information given about potentially serious side effects. Weigh scales (if required), instruction and daily weigh charts provided with instructions when to contact researchers Social worker and the home care team visited patient to facilitate discharge planning and identify and manage potential economic, social or transport problems. Discharge summary to home care team prepared by study nurse. After discharge: Home care team nurse visited within 48 hrs (usually within 24 hours): physical assessment of patient, reinforced teaching, reviewed medication and lifestyle advice, assisted with initiating daily weights. Two further home care team nurse visits in first week. Study nurse phoned patient at home to assess progress and answer questions. Patients encouraged to contact own doctors or study team with problems or questions Educational visits covered: CHF diagnosis, symptoms, treatment, follow up and prognosis using educational booklet developed by the researchers Comparison Group: usual care “Conventional medical care as determined by the patient’s usual physician,” this could include social service evaluation, dietary and medication teaching, home care and all other available hospital services |
|
| Outcomes | Primary endpoints: Unplanned re-admissions within 90 days of discharge. Total number of days hospitalised during follow up. Also looked at: Re-admission rates in the moderate risk subgroup compared to the high risk sub group Analysis done on intention to treat basis?: yes* |
|
| Notes | Data source: published data and information from author*. Generalisability: Number of patients screened for HF not given, 261 patients fulfilled criteria for CHF, 21 (8%) died during initial hospitalisation, 188 (72%) classified as intermediate or high risk, 67 (36% of 188) excluded, 23 (12% of 188) patient or physician refused, 98 (52% of 188) participated Consort flow chart: not applicable, before 1996. Rationale for sample size: not given, pilot study. Other points: Reasons for exclusions (67/188 patients): residence outside hospital catchment 34%; planned discharge to a nursing home or other chronic care facility 22%; non-cardiac illness likely to result in non-preventable re-admission, mental incapacity or psychiatric disturbance 12%; logistic and discretionary reasons 31% Not clear if some of the patients who died in hospital were originally randomised into the study or not. Generation of randomisation sequence: computer generated list of random numbers* Allocation concealment: “The principal investigator and study statistician were blinded; the patients and nurses were not blinded.”* Risk of care giver performance bias: possible, care providers were not masked. Risk of attrition bias: low. Risk of detection bias: high, “detailed data were collected for all patients by a research nurse and study physician”, although those doing the analyses were masked as to the patient’s allocation* |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | RCT, single centre Recruitment: July 1990 to June 1994 (main study), sub study subjects enrolled in the 2nd and 3rd years of the trial. Duration of follow up: 90 days after discharge. |
|
| Participants | Country: USA Participants in main study: 140 patients (57 males, 41%) in comparison group, 142 (46 males, 32%) in intervention group. (Sub study of medication compliance: 76 patients (41% males) in comparison group, 80 (26% males) in intervention group). Actual age of study subjects (main study): median age 79 years (range not given). Male sex: 37%. Ethnicity: ‘white’ 45%. Actual severity of heart failure in study subjects at recruitment (main study): NYHA class, means: control group 2.4 (SD 1.1), intervention group 2.4 (SD 1.0) LVEF (on 222 patients, 79%): control group mean 41% (SD 13), intervention group 44% (SD 14) Study inclusion criteria:
Study exclusion criteria: “Patients defined as low risk for re-admission based on the absence of each of the four independent risk factors for re-admission listed above. “Anticipated survival < 3 months. “Residence outside hospital catchment area. “Planned discharge to a nursing home or other chronic care facility. “Severe dementia. “Severe psychiatric illness. “Physician refusal. “Logistic and discretionary reasons (including participation in the 1993 feasibility study) |
|
| Interventions | Duration of intervention: unclear - mostly during index admission and first week after discharge but telephone contact maintained to 90 days? Intervention Group: ‘Nurse directed multidisciplinary intervention’ Same intervention as pilot study: see Rich et al (1993) above Comparison Group: usual care Eligible to receive all standard treatment and services ordered by their primary physicians, no standard or generally accepted treatment withheld |
|
| Outcomes | Primary endpoint: Survival for 90 days without re-admission Secondary endpoints: All cause re-admissions. Heart failure re-admissions. Total number of days in hospital during follow up. QOL at baseline and 3 months in subset of 126 patients using Chronic heart failure Questionnaire Costs, medical and caregiver costs collected prospectively with cost logs for 57 patients during final year of the study Sub study of medication compliance endpoints: Average percentage of pills taken correctly for each medication at 30 days after discharge. Total number of pills taken correctly divided by total number of pills that should have been taken Analysis done on intention to treat basis? (main study): yes |
|
| Notes | Data source: published data and information from author*. Generalisability: Number of patients screened for HF not given, 1306 patients fulfilled criteria for CHF, 915 (70%) classified as moderate or high risk for re-admission, 517 (57% of 915) excluded, 116 (13% of 915) patient or physician refused, 282 (31% of 915) participated Consort flow chart: not applicable pre 1996 Rationale for sample size: given in feasibility study, Rich et al (1993) Other points: Reasons for exclusions (517 patients): residence outside hospital catchment 27%; planned discharge to a nursing home or other chronic care facility 22%; terminal illness 13%; dementia or psychiatric illness 4%; logistic and discretionary reasons 34% Not clear how heart failure related admissions were determined Generation of randomisation sequence and allocation concealment: “The patients underwent blinded randomisation with the use of a computer-generated list of random numbers after consenting to participate in the study. Neither the patient nor the members of the study team were aware of the treatment assignment until after randomisation.” Risk of care giver performance bias comment: possible, care providers were not masked*. Risk of attrition bias: low. Risk of detection bias: high, study team collected some of the endpoint data, although medication and dietary compliance data collection was masked |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Cluster RCT, clusters randomised at level of provider sites. Index admissions at two hospitals. Recruitment period: not given. Duration of follow up: six months |
|
| Participants | Country: USA Participants: 228 control group and 130 intervention patients enrolled; data on resource use and satisfaction available on a subsample of 105 males (46%) in control group , 130, 70 males (54%) in intervention group. Actual age of study subjects mean (SD): control group 74.6 (12), intervention group 72.5 (13.1) Sex: 49% male Ethnicity: not given. Actual severity of heart failure in study subjects at enrolment: NYHA class, n (%): controls II (4%), III (38%), IV (58%) intervention group II (2%), III (36%), IV (62%) Study inclusion criteria:
Study exclusion criteria:
|
|
| Interventions | Duration of intervention: six months Intervention Group: Telephonic case management After discharge: Telephonic case management by a registered nurse using a decision-support software program developed by Pfizer. Patient telephoned at home by case manager (a registered nurse) within five days of hospital discharge ‘and thereafter at a frequency guided by software and case manager judgement based on patient symptoms, knowledge and needs’. The case manager also spoke with family members and consulted community agents and other professionals (e.g. physicians, dieticians, social workers and physical therapists). Patients were sent printed educational material monthly. Physicians were sent guidelines on HF management and automated reports on patient progress and phoned by case managers as required The software emphasised poor adherence to medication and dietary regimens and lack of patient knowledge of signs and symptoms of clinical deterioration; and set priorities for patient education and data collection. The software used best practices derived from published guidelines and evidence Comparison Group: usual care. Care not standardised, no further details given. |
|
| Outcomes | Primary endpoint: HF hospitalisation rate (Mean number of admissions with HF per patient) Other endpoints: All cause hospitalisation rate. HF readmission rate (proportion readmitted). All cause readmission rate. HF hospital days. All cause hospital days. Mean time to rehospitalisation. Multiple readmissions. Emergency department visits. Physician office visits Patient satisfaction. Also looked at: Cost of acute care Analysis done on intention to treat basis? No |
|
| Notes | Data source: published data only Generalisability: 1145 hospitalised patients thought to have heart failure were screened; 573 (50%) were eligible for the study; 148 (26%) refused consent; 29 (5%) patient’s physicians refused consent; 28 withdrew during the course of the study (5%); 10 (2%) were dropped for logistical reasons; and 358 (62%, 31% of those screened) of these were randomised Consort flow chart: not supplied Rationale for sample size: given, but no mention of intra-cluster correlation coefficient. In addition although they have given correct sample size for a normally distributed variable, hospitalisation rate is not normally distributed. The researchers note this problem, but their solution (logarithmic transformation) may not resolve it Other points: Study funded by Pfizer Inc. Decision support computer software programme developed by Pfizer Inc and one author employed at Pfizer inc Generation of randomisation sequence and allocation concealment: no information provided. Comment on risk of care giver performance bias: unlikely Risk of attrition bias: unclear Risk of detection bias: unclear, Data were extracted from records in treatment centres by nurses. It is not clear what ‘a single provider site’ is this one hospital or one part of a hospital. Very uneven groups: 130 participants in intervention group and 228 in the intervention group, reasons unclear. Intervention patients received an average of 17 phone calls (median 14, inter quartile range 11-22) in the six month follow up period |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | RCT, single centre Recruiting: March 1997 to May 1998 Duration of follow up: 6 months |
|
| Participants | Country: Australia Participants: 100 patients (59 males) in comparison group, 100 (65 males) in intervention group, Actual age of study subjects: control group mean 76.1 years (SD 9.3), intervention group 75.2 years (SD 7.1) years Male sex: 62% Ethnicity: not given Actual severity of heart failure in study subjects at recruitment: NHYA class, n,: control group II 48, III 43, IV 9, intervention group II 42, III 46, IV 12 LVEF: control group mean 37% (SD 11), intervention group 37% (SD10) Study inclusion criteria:
Study exclusion criteria:
|
|
| Interventions | Duration of intervention: mainly within 2 weeks of discharge but some phone contact throughout study Intervention Group: Usual care plus ‘Multidisciplinary, home-based intervention’ After discharge: Comprehensive assessment at home by a cardiac nurse 7-14 days after discharge. After home visit nurse sent report to primary care physician and cardiologist. Cardiac nurse arranged a flexible diuretic regimen for patient’s weight and symptoms if required. Phone call by cardiac nurse to patient contact at 3 and 6 months. Patients encouraged to contact the nurse if any problems arose. Home visits repeated if a patient had two or more unplanned re-admissions within 6 months of index admission Home visit included: assessment of clinical status, physical activity, adherence to medication, understanding of disease, psychosocial support and use of community resources. Followed by (as appropriate): ‘remedial counselling’ to patients and their families, strategies to improve adherence, simple exercise regimen, incremental monitoring by family/carers, urgent referral to 10 care physician. (Median duration of visit = 2 hr (range 1-3.5hr)). Comparison Group: usual care All study patients could be referred to cardiac rehab nurse, dietician, social worker , pharmacist and community nurse as appropriate. All patients had appointment with their primary care physician and/or cardiology outpatient service within 2 weeks of discharge. Regular outpatient review by the cardiologist was undertaken throughout the follow up period |
|
| Outcomes | Primary endpoint: Frequency of unplanned re-admissions plus all cause out-of-hospital deaths (i.e. composite endpoint) during 6 months follow up Other endpoints: Time to first primary endpoint (event-free survival). Frequency of unplanned re-admissions. Days of unplanned re-admissions. All cause deaths. Out of hospital deaths. Cost of hospital and community based health care sample of patients only) Random sample of patients only: Minnesota living with heart failure questionnaire and Australian version of SF-36 at baseline, 3 & 6 months All analyses in first study done on intention to treat basis |
|
| Notes | Data source: published data only Generalisability: Of 4055 cardiology inpatients screened over 14 month period only 285 (7%) were clinically eligible, 200 (70%, 5% of 4055) participated, 59 (21%) met at least one exclusion criteria and 26 (11%) refused consent or died before discharge Consort flow chart: supplied Rationale for sample size: given Other points: Reasons for exclusions: proportions of patients with different reasons for exclusion not given Generation of randomisation sequence and allocation concealment: “Telephone call to an investigator who was unaware of the patient’s demographic and clinical profile, who then allocated the individual [to group] via a computer generated protocol.” Risk of care giver performance bias: high, as part of the intervention patient’s primary care physician and cardiologist received a report on the patients’ home assessment and any actions taken or recommended. Risk of attrition bias: low Risk of detection bias: low - all data collection and analysis was done “with masking maintained” assume means they were masked to patients’ intervention/usual care group status |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
CHF = chronic heart failure, HF = heart failure, LOS = length of stay, HRQL = health related quality of life, NYHA = New York Heat Association functional class, PVD = peripheral vascular disease, LVEF = left ventricular ejection fraction, LV = left ventricle, Tx = transplantation, i/v = intravenous, Rx = therapy, MI = myocardial infarction, Q= questionnaire, ACEI= angiotensin converting enzyme inhibitor, GP = general practitioner
* = information obtained from personal communication with author
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Akosah 2002 | Non-randomised study. |
| Azevedo 2002 | Non-randomised study. |
| Barth 2001 | Very small RCT, limited data presented,statistical analyses appear incorrect |
| Benatar 2003 | RCT both arms received an intervention. |
| Cordisco 1999 | Non-randomised study |
| Costantini 2001 | Mixed before and after and parallel group study. |
| de Lusignan 1999 | Hospital admission for heart failure not an inclusion criterion |
| Evans 1993 | “Generic intervention” (ie not exclusively designed for, or directed at, patients with CHF) |
| Farag 1967 | Non-randomised study. |
| Fitzgerald 1994 | “Generic” intervention |
| Gattis 1999 | Hospital admission for heart failure not an inclusion criterion |
| Goodyer 1995 | Hospital admission for heart failure not an inclusion criterion |
| Grancelli 2003 | Hospital admission for heart failure not an inclusion criterion |
| Hanchett 1967 | Hospital admission for heart failure not an inclusion criterion |
| Hansen 1992 | “Generic” intervention |
| Heidenreich 1999 | Non-randomised study. |
| Hughes 2000 | “Generic” intervention |
| Jerant 2001 | Small RCT with three arms: 13 patients receiving home tele care; 12 patients received telephone care; 12 received usual care. An interesting paper but excluded form this review because the presentation and analyses of these data do not allow either of the two interventions to be compared with the control treatment |
| Johnson 2000 | “Generic” intervention |
| Lin 2001 | Non-randomised study. |
| Naylor 1994 | “Generic”’ intervention. |
| Naylor 1999 | “Generic”’ intervention. |
| Philbin 2000 | RCT, unit of randomisation was the hospital, analyses of outcome at hospital level only |
| Riegel 2000 | Non-randomised study. |
| Rubin 1992 | “Generic” intervention. |
| Schneider 1993 | Non-randomised study. |
| Serxner 1998 | Purely educational intervention |
| Stewart 1998a | “Generic” intervention. |
| Stewart 1998b | Subgroup from a ‘“generic” study. |
| Stewart 1999b | Subgroup from a ‘“generic” study. |
| Stewart 2002b | Follow up data at 4.2 years combining data from included study (Stewart 1999 Lancet) and excluded study (Stewart 1998 JAGS). Data on included study not presented separately |
| Topp 1998 | Non-randomised study. |
| Townend 1988 | “Generic” intervention. |
| van Rossum 1993 | “Generic” intervention. |
| Weinberger 1996 | “Generic” intervention. |
| Williams 1994 | “Generic” intervention. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The evaluation of a nurse-led intervention to improve self-management for patients admitted to hospital with a diagnosis of heart failure (due to left ventricular systolic dysfunction) |
| Methods | |
| Participants | 250 patients (125 in intervention arm, 125 in control arm) |
| Interventions | The intervention is designed to enhance patients’ sense of self efficacy (confidence) in their ability to adhere to medication and other aspects of their treatment regime including fluid restriction, diet exercise and self monitoring for signs of deteriorating heart failure, using a problem solving approach |
| Outcomes | Primary endpoints: All cause hospital re-admissions and heart failure related hospital re-admissions during the first three months after discharge. Numerous secondary endpoints including mortality and 12 month data |
| Starting date | NA, study likely to be completed in 2005. |
| Contact information | Dr. Suzanna Hardman Consultant Cardiologist with an interest in Community Cardiology, The Whittington & UCL Hospitals, Clinical & Academic Department of Cardiovascular Medicine, St Mary’s Wing, Whittington Hospital, Highgate Hill, London N19 5NF, UK |
| Notes |
| Trial name or title | A controlled trial of heart failure management programs |
| Methods | |
| Participants | 147 patients with symptomatic CHF at 5 VA facilities |
| Interventions | Three groups: usual care, nurse manager, home monitoring also in two sites patients randomised to HF clinic |
| Outcomes | Death or hospitalisation for a cardiac cause |
| Starting date | NA |
| Contact information | NA |
| Notes | Poster abstract only. Author contacted, full trial not published yet |
| Trial name or title | Community case management decreases rehospitalisation rates and costs and improves quality of life in heart failure patients with preserved and non-preserved left ventricular function: a randomised controlled trial |
| Methods | |
| Participants | 136 patients |
| Interventions | Community case management: a home visit and weekly phone calls for one month followed by monthly phone calls from a HF nurse aimed at patient assessment, comprehensive education and counselling |
| Outcomes | Hospitalisations, health care costs, LOS in hospital, QOL. |
| Starting date | NA |
| Contact information | Prof. Debra Moser, College of Nursing, Ohio State University, Columbus, Ohio USA |
| Notes |
| Trial name or title | Nursing case management for elderly heart failure patients |
| Methods | |
| Participants | 200 patients aged 65 years or older hospitalised at one centre for the treatment of CHF |
| Interventions | Intervention group receive enhanced discharge planning, and are taught to manage their CHF within parameters set by their physician using a workbook for guidance. In addition they receive patient-specific printed material and ongoing assessment and follow up by a nurse for a 6 month period through phone calls and visits |
| Outcomes | Morbidity, mortality, quality of life and functional status at 6 months and one year after discharge |
| Starting date | NA, in July 1998 57 patients had been recruited. |
| Contact information | NA |
| Notes |
| Trial name or title | TEN-HMS: Trans European Network-Homecare Monitoring Study |
| Methods | |
| Participants | 427 patients |
| Interventions | Three groups: usual care, monthly phone calls from nurse, home telemonitoring |
| Outcomes | Event free survival |
| Starting date | NA, study completed |
| Contact information | NA |
| Notes |
| Trial name or title | Effect of a home-based intervention in patients with heart failure (RCT) |
| Methods | |
| Participants | 160 patients with CHF with <55% LEVF (echocardiography or nuclear scan) |
| Interventions | Usual care vs. nurse-led home-based and hospital outpatient department-based intervention in patients with CHF involving education, support, pharmacotherapy and monitoring |
| Outcomes | All deaths, all cause hospital admissions, quality ‘of life. |
| Starting date | 1st January 2001 |
| Contact information | Prof. David R. Thompson, Department of Health Sciences, University of York, Genesis 6, York Science Park, Heslington, YorkY010 5DQ, UK |
| Notes | Not clear that all patients have had a previous hospital admission for heart failure so study may not be eligible for inclusion in this review |
DATA AND ANALYSES
Comparison 1. Case management vs. usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at any length of follow up (all studies, ordered by duration of follow up) | 10 | 1799 | Odds Ratio (M-H, Fixed, 95% CI) | 0.86 [0.67, 1.10] |
| 2 Mortality at follow up (studies where allocation concealment confirmed) | 4 | 663 | Odds Ratio (M-H, Fixed, 95% CI) | 0.69 [0.46, 1.03] |
| 3 Mortality at follow up (studies appearing to be of reasonable or high quality using Delphi criteria) | 5 | 751 | Odds Ratio (M-H, Fixed, 95% CI) | 0.68 [0.46, 0.98] |
| 4 Readmitted with heart failure by end of follow up (all studies with data) | 7 | 1051 | Odds Ratio (M-H, Fixed, 95% CI) | 0.52 [0.39, 0.70] |
Comparison 2. Clinic vs. usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at follow up | 2 | 355 | Odds Ratio (M-H, Fixed, 95% CI) | 0.95 [0.57, 1.57] |
Comparison 7. Intervention vs. control, case management studies only (all).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at end of follow up period (all durations of follow up period) | 0 | 0 | Odds Ratio (M-H, Fixed, 95% CI) | Not estimable |
Analysis 1.1. Comparison 1 Case management vs. usual care, Outcome 1 Mortality at any length of follow up (all studies, ordered by duration of follow up).
Review: Clinical service organisation for heart failure
Comparison: 1 Case managementvs. usual care
Outcome: 1 Mortality at any length of follow up (all studies, ordered by duration of follow up)
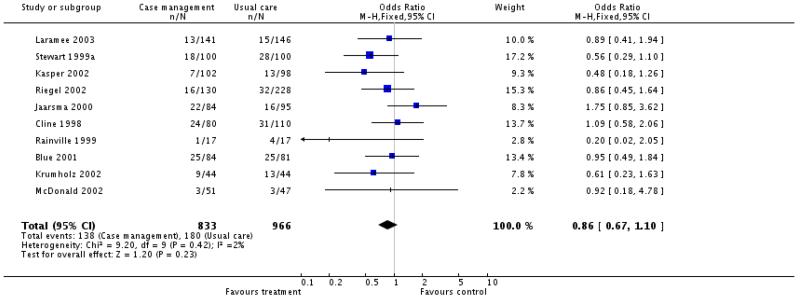
|
Analysis 1.2. Comparison 1 Case management vs. usual care, Outcome 2 Mortality at follow up (studies where allocation concealment confirmed).
Review: Clinical service organisation for heart failure
Comparison: 1 Case managementvs. usual care
Outcome: 2 Mortality at follow up (studies where allocation concealment confirmed)
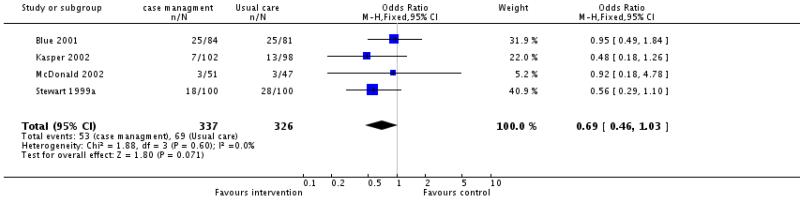
|
Analysis 1.3. Comparison 1 Case management vs. usual care, Outcome 3 Mortality at follow up (studies appearing to be of reasonable or high quality using Delphi criteria).
Review: Clinical service organisation for heart failure
Comparison: 1 Case managementvs. usual care
CUitcom e: 3 M ortality at follow up (studies appearing to be of reasonable or high quality using Delphi criteria)
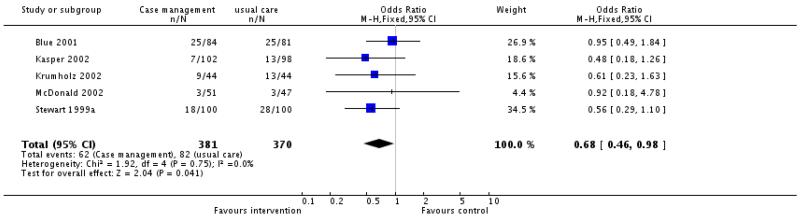
|
Analysis 1.4. Comparison 1 Case management vs. usual care, Outcome 4 Readmitted with heart failure by end of follow up (all studies with data).
Review: Clinical service organisation for heart failure
Comparison: 1 Case management vs. usual care
Outcome: 4 Readmitted with heart failure by end of follow up (all studies with data)
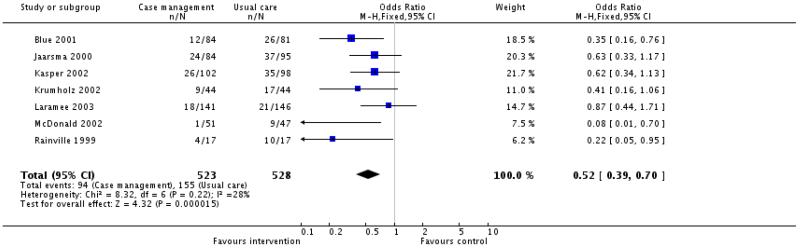
|
Analysis 2.1. Comparison 2 Clinic vs. usual care, Outcome 1 Mortality at follow up.
Review: Clinical service organisation for heart failure
Comparison: 2 Clinic vs. usual care
Outcome: 1 Mortality at follow up
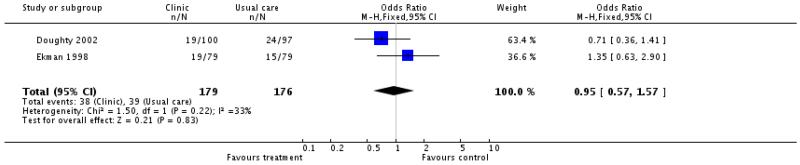
|
ADDITIONAL TABLES
Table 1. Electronic Searches.
| Database | Search Strategy |
|---|---|
| Cochrane Register of Controlled Trials (CENTRAL/ CCTR) and DARE | HEART-FAILURE-CONGESTIVE*:ME (HEART near FAILURE) (CARDIAC near FAILURE) ((#1 or #2) or #3) PATIENT-CARE-MANAGEMENT*:ME HOME-CARE-SERVICES*:ME (PATIENT near CARE) (HOME near INTERVENTION) (HOME near CARE) REHABILITAT* (SECONDARY near PREVENT*) NURS* MULTIDISCIPLIN* EXERCISE PHYSICAL-FITNESS*:ME EXERCISE-THERAPY*:ME (PHYSICAL near ACTIVITY) (PHYSICAL near TRAIN*) (PHYSICAL near FIT*) (STRENGTH near TRAIN*) (AEROBIC near TRAIN*) (RESISTANCE near TRAIN*) (((((((((#5 or #6) or #7) or #8) or #9) or #10) or #11) or #12) or#13) or #14) (((((((#15 or #16) or #17) or #18) or #19) or #20) or #21) or # 22) (#23 or #24) (#4 and #25) |
| MEDLINE |
|
| EMBASE | “Congestive heart failure/ or “congestive heart failure”.mp. “Heart failure/ or “heart failure”.mp. “Heart failure/ or “cardiac failure”.mp. “1 and 2 and 3 ”“PATIENT CARE MANAGEMENT”.mp. ““HOME CARE SERVICES”.mp. “Patient care/ or “patient care”.mp. ““DELIVERY CARE”.mp. ““HOME INTERVENTION”.mp. “Home care/ or “home care”.mp. ““HOMECARE”.mp. “Rehabilitation/ or “rehabilitation”.mp. “Secondary prevention/ or “secondary prevention”.mp. ““##‘Nurs#’.mp.##”/ “Nursing/ or “nursing”.mp. ““MULTI DISCIPLINARY”.mp. ““HOME VISIT”.mp. ““HOME ASSESSMENT”.mp. “Primary medical care/ or “primary care”.mp. ““PATIENT MANAGEMENT”.mp. ““DISCHARGE PLANNING”.mp. ““PATIENT CARE PLANNING”.mp. ““PATIENT CARE TEAM”.mp. ““HOUSE CALLS”.mp. “Exercise/ or “exercise”.mp. ““PHYSICAL FITNESS”.mp. ““EXERCISE THERAPY”.mp. “Physical activity/ or “physical activity”.mp. ““PHYSICAL TRAINING”.mp. ““STRENGTH TRAINING”.mp. ““AEROBIC TRAINING”.mp. ““RESISTANCE TRAINING”.mp. “5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 “1 or 2 or 3 “33 and 34 “from 35 keep 1-8 “from 35 keep 1-501 |
| CINAHL |
|
| AMED |
|
| CVD Trials Register at McMaster | (Title = “heart failure” or “cardiac failure” OR Keywords = “heart failure” or “cardiac failure” OR #43 = “heart failure” or “cardiac failure” ) AND (Title = home* OR care* OR plan* or manag* OR Keywords = home* OR care* OR plan* OR manag* OR #43 = home* OR care* OR plan* or manag*) |
| Science Citation Index Expanded | Forward search for papers citing the following references: Weinberger 1996; Stewart 1998a; Rich 1995. |
| SIGLE | Search History * #4 #1 or #2 or #3 (87 records) #3 cardiac near failure (13 records) #2 heart near failure (80 records) #1 heart failure (79 records) |
Table 2. Study components (as indicated in published reports).
| Study | Phone f/u | Largely Educational |
Self management |
Weight monitoring |
Dietary advice |
Exercise promotion |
Medication review |
Social/ psych. sup. |
Duration |
|---|---|---|---|---|---|---|---|---|---|
| Rich 1993 | Y | Y | Y | Y | Y | Y | Y | Not clear, at least one week after discharge | |
| Rich 1995 | Y | Y | Y | Y | Y | Y | Y | Not clear, at least one week after discharge | |
| Cline 1998 | Y | Y | Y | 12 months | |||||
| Eckman 1998 | Y | Y | Y | Y | Not clear, mean follow up time was 5 months | ||||
| Rainville 1998 | Y | Y | Y | 3 months | |||||
| Stewart 1999 | Y | Y | Intervention concentrated in first 2 weeks 2 weeks but some phone contact up to end of follow up (6 months) | ||||||
| Jaarsma 2000 | Y (one call) | Y | Around one week | ||||||
| Blue 2001 | Y | Y | Y | Y | Up to 12 months | ||||
| Capomollo 2002 | Y | Y | Y | Y | Y | Y | Not clear | ||
| Doughty 2002 | Y | Y | Y | Y | Y | 12 months | |||
| Harrison 2002 | Y (one call) | Y | Y | Y | Y | ? | Two weeks following discharge | ||
| Kasper 2002 | Y | Y | Y | Y | 6 months | ||||
| Krumholz 2002 | Y | Y | 12 months | ||||||
| McDonald 2002 | Y | Y | Y | Y | 3 months | ||||
| Riegel 2002 | Y | Y | 6 months | ||||||
| Laramee 2003 | Y | Y | Y | Y | Y | Y | 3 months |
Table 3. Results of Included Studies.
| Study ID | Results | Notes |
|---|---|---|
| Rich 1993 | (All reported as intervention gp. vs. control gp.) Primary endpoints: Proportion readmitted within 90 days: 21/63 (33.3%, 95% CI 21.7, 44.9) vs. 16/35 (45.7%, 95% CI 29.2, 62.2) NS (Not clear if this is unplanned re-admissions or all re-admissions). Total number of days hospitalised within 90 days: mean 4.3 days per patient (SE 1.1, 95% CI 2.1, 6.5) vs. 5.7 (SE 2.0, 95% CI 1.8, 9.6) NS, P value not given. Also looked at: Days to first re-admission: mean 31.8 days (SE 5.1, 95% CI 21.8, 41.8) vs. 42.1 (SE 7.3, 95% CI 27.8, 56.4) NS, P value not given. Proportion readmitted within 90 days in the moderate risk subgroup: 11/40 (27.5%, 95% CI 18.7, 41.3) vs. 10/21 (47.6%, 95% CI 26.2, 69.0) NS P = 0.1, Fisher’s exact test Proportion readmitted within 90 days in the high risk subgroup: 10/23 (43.5% 95% CI 23.2, 63.8) vs. 6/14 (42.9%, 95% CI 17.0, 68.8) NS, P value not given. Total number of days hospitalised within 90 days in the moderate risk subgroup: 3.2 (SE 1.2, 95% CI 0.8, 5.6) vs. 6.7 (SE 3.2, 95% CI 0.4 to 13.0) NS, P value not given. Total number of days hospitalised within 90 days in the high risk subgroup: 6.3 (SE 2.3, 95% CI 2.0 to 10.6) vs. 4.2 (SE 1.4, 95% CI 1.5 to 6.9) NS, P value not given. Days to first re-admission in the moderate risk subgroup: 35.1 (SE 9.0, 95% CI 17.5 to 52.7) vs. 28.6 (SE 7.2, 95% CI 14.5, 42.7) NS, P value not given. Days to first re-admission in the high risk subgroup: 27. 8 (SE 3.5, 95% CI 20.9 to 34.7) vs. 60.2 ( SE 10.5, 95% CI 39.6, 80.8) P < 0.05 in favour of usual care, Mann-Whitney rank sum test |
Comment on statistical analyses: Since it was a feasibility study this study was not powered to show differences in its primary endpoints. Overall 38% (95% CI 28 to 47) of patients were readmitted within 90 days. No information on survival supplied, all patients were followed up for 90 days - presumably all survived |
| Rich 1995 | (All reported as intervention gp. vs. control gp.) Primary endpoint Survival for 90 days without re-admission: 91 (64.1%) vs. 75 (53.6%), absolute difference 10.5% (95% CI −0. 9, +21.9) P = 0.09, NS Secondary endpoints Total number of readmissions in 90 days (all cause): 53 vs. 94, P = 0.02, Wilcoxon rank-sum test Patients with at least one re-admission in 90 days (all cause): 41 (28.9%) vs. 59 (42.1%), absolute difference 13.2% (95% CI 2.1, 24.3), P = 0.03 Patients with more than one re-admission in 90 days (all cause): 9 (6.3%) vs. 23 (16.4%), absolute difference 10.1% (95% CI 2.8, 17.4), P = 0.01 Total number of days in hospital during follow up: 556 vs. 865 Mean days in hospital during follow up per patient: 3. 9 (SD 10.0) vs. 6.2 (SD 1.4), P = 0.04, Wilcoxon rank-sum test Total number of heart failure re-admissions in 90 days: 24 vs. 54, P = 0.04, Wilcoxon rank-sum test QOL (CHFQ) at baseline and 3 months in subset of 126 patients (67 intervention patients, 59 control): Total score mean change between baseline and 90 days +22.1 ( SD 20.8) vs. +11.3 (SD 16.4) P 0.001 (QOL also increased significantly more in the intervention group compared to the control group in each of the 4 subscales of the CHFQ) Costs of care for study patients: $4,815 mean costs per patient vs. $5,275 Also reported Deaths in follow up period: 13 (12.1%) vs. 17 (9.2%), NS Admission to long term care facility during follow up: 5 vs. 6 Survival for 90 days without re-admission in those surviving initial hospitalisation: 66.9% vs. 54.3 6%, absolute difference 12.6% (95% CI 1.1, 24.1) P = 0.04 Re-admission rates in 9 months after follow up (all cause): NS difference Heart failure re-admission rates in 9 months after follow up: 57 vs. 80, P 0.08, NS Substudy of medication compliance: Average percentage of pills taken correctly for each medication at 30 days after discharge (method 1): 87.9% (SD 12.0, range 33.3% to 100%) vs. 81.1 (SD 17.2, range 23.1% to 100%), P 0.003 Total number of pills taken correctly divided by total number of pills that should have been taken (method 2): 87.5% (SD 12.6, range 35.1 to 100%) vs. 80.9 (Sd 16.7, range 23% to 100%) Overall mean compliance rate for sub study population: method 1, 84.6% (SD 15.1, range 23.1% to 100%); method 2, 84.3% (SD 15, range 23% to 100%), P = 0. 004 |
Comment on statistical analyses: The study had adequate power - with 282 patients it was the largest heart failure specific study identified in this review and statistical review suggests that all statistical analyses were appropriate. Not clear how heart failure re-admissions and non CHF re-admissions were distinguished. Not clear how similar two groups are at baseline in subset of patients with QOL data. Not clear whether the cost data was just on 57 patients and how those patients were selected and how similar the two groups were at baseline |
| Cline 1998 | (All reported as intervention gp. vs. control gp.) “Main outcomes” (primary endpoint not specified): Mean days to re-admission in survivors at one year: 141 (87) vs. 106 (101), P < 0.05 (see comment below). Mean days in hospital in survivors at one year: 4.2 (7. 8) vs. 8.2 (14.7) NS, P= 0.07 (unequal variance). Health care costs in survivors at one year: (mean costs) 2294 US$ vs. 3594 US$, NS, P= 0.07. Also looked at: Deaths at one year: 24 (31%) vs. 31 (28%), NS, (test statistic not given) Deaths at 90 days (Cline 2001): 8/80 vs. 17/110, P given as <0.001, erroneous, our estimation P = 0.3 Death or at least one re-admission to hospital (composite end point) at 12 months: 56 (70%) patients vs. 79 (72%) patients, NS, (test statistic not given) Death or at least one re-admission to hospital (composite end point) at 90 days: At three months 53 (66%) vs. 61(56%) NS Number of patients surviving to one year who were readmitted: 22 (39%) vs. 43 (54%) NS, P =0.08 Mean no. of hospitalisations per patients surviving to one year: 0.7 (SD 1.1) v 1.1 (SD 1.8) NS , P =0.08 Outpatient visits (not clear how defined): 3.6 (3.2) vs. 4.0 (3.4) NS Treatment at one year, % on ACEI: 41 (75%) vs. 41 (52%) P <0.05 Treatment at one year, % on all other HF drugs: all differences NS The quality of life in heart failure questionnaire, Nottingham health profile and patients’ global self assessment: all differences NS at one year, (test statistics not given) |
Comment on statistical analyses: No sample size calculation given, but our post hoc calculations suggest that this study had adequate power. We note an apparent error on page 444, the text says that says 56/79 patients died or were readmitted, Table 2 says 56/79 patients survived some with readmission. Elsewhere the text suggests 46/74 died or were readmitted. The mean time to re-admission in patients who survived to one year was longer in the intervention than in the control group (mean days to re-admission 141 (SD 87) vs. 106 (100) is given as having P <0.05, but this result is contradicted by the non-significant result of the robust log-rank test which tests the outcome time to death or readmission. Outcome data on re-admissions, days in hospital and costs all on survivors at one year not on whole group. Health care costs involved many assumptions. Study had some before and after analyses that are not reported here |
| Ekman 1998 | (All reported as intervention gp. vs. control gp., where appropriate.) Main endpoints: Proportion of patients aged >65 years who were eligible for the study: 15% (95% CI 13, 17) NB this included those who refused. Proportion of patients in the intervention group who did not visit the HF nurses: 23 (29%, 95% CI 19-39%) (11 died, 11 fatigued/unwilling, 1 institutionalised) Functional class: change in NYHA classification between baseline and 6 month follow up int. gp. - cont. gp. = 0.08 (95% CI −0.19, 0.35), NS (test statistic not given) Hospitalisations: mean difference intervention gp. -control. gp. = −0.1 (95% CI −0.5 to 0.3), NS (test statistic not given) Deaths: 19 (24%) vs. 15 (19%), NS (test statistic not given) Hospital days: intervention gp. - control. gp. difference in mean days 8 (SD 26), difference in median days 4, P= 0.29 (NS) Mann-Whitney U test Also reported: Survival at 6 months without re-admission: 25 (32%) vs. 31 (39%), NS (test statistic not given) 7 (9%) intervention patients made spontaneous visits to HF nurses as a result of clinical deterioration and were admitted to hospital. 4 (median) phone contacts with nurses per intervention patient. 22 (28%) intervention patients made spontaneous phone contacts to HF nurses. 18 carers/relatives (23%) of intervention patients made spontaneous phone contacts to HF nurses |
Comment on statistical analyses: Since there were only 79 patients in each arm of this feasibility study it may have lacked the statistical power to detect differences in its main outcomes. No sample size calculation was given (feasibility study). |
| Rainville 1999 | (All reported as intervention group. vs. control group.) Primary endpoint: Number of patients who died (all causes) or were read-mitted with HF at one year: 5 vs. 14, P < 0.01 probably Chi squared test (NB See statistical comment below.) Time to re-admission for HF or patient death: Significantly longer in intervention group, P < 0.01 log rank test. Also reported: Number of patients readmitted with HF at one year : 4 vs. 10, P 0.05 probably Chi squared test (NB See statistical comment below.) Deaths (all cause) at one year: 1 v 4 (test statistic not given) Total no. of re-admissions at one year: 20 v 26 NS, (test statistic not given) Change in functional health assessment score (Dartmouth COOP charts): no significant change at 30 or 90 days for either group (no test results given) |
Comment on statistical analyses: Very small sample size. Inappropriate statistical tests used with small sample sizes. (Chi-square test should not be used here, the correct test is Fisher’s exact test.) |
| Stewart 1999a | (All reported as intervention gp. vs. control gp.) Primary endpoint: Frequency of unplanned re-admissions plus all cause out-of-hospital deaths during 6 months follow up: 77 primary events vs. 129 primary events, event rates per month 0.20 (95% CI 0.14-0.26) vs. 0.40 (0.24-0.56), P = 0.02 (test not clear) (NB see statistical comment). Other endpoints: Frequency of unplanned re-admissions alone at 6 months: 68 vs. 118, event rates per month 0.14 (95% CI 0.10-0.18) vs. 0.34 (0.19-0.49), P = 0.03 (NB see statistical comment). Out of hospital deaths at 6 months: 9 v. 11, NS All cause deaths at 6 months: 18 vs. 28, P = 0.098 Number of patients remaining event free (i.e. death or re-admission) at 6 months: 51 vs. 38, P = 0.04 Total unplanned days in hospital at 6 months: 460 v. 1174, event rates per month 0.9 (0.6-1.2) vs. 2.9 (1.93.9), P = 0.01(NB see statistical comment). Total hospital based costs (including inpatient, out patient and emergency services): median per patient per month, A$ 252 [IQR 37-1179] vs. 438 [42-2172], P = 0.16. Cost of hospital and community based health care, (sample of patients only): median per patient per month, A$ 400 vs. 380, P = 0.91. Change in Minnesota living with heart failure questionnaire between baseline and 3 & 6 months (random sample of 68 patients): intervention group significantly bigger fall in score than control group at 3 months (higher scores indicate impaired QOL but clinical significance of change seen not clear), no significant difference in scores of survivors at 6 months. Change in Australian version SF-36 between baseline and 3 & 6 months (random sample of 68 patients): no differences seen in mental health scores, change in physical health scores at 3 months significantly higher in intervention group (clinical significance not clear) but no difference in survivors at 6 months. Also looked at: Difference in probability of survival at 18 months: P = 0.1 Frequency of unplanned re-admissions alone at ‘the end of follow up’ (around 18 months): 118 vs. 156, event rates per month 0.15 (0.11-0.19) vs. 0.37 (0.19-0.55), P = 0.053 Total elective days in hospital at 6 months: 87 vs. 25, P = 0.13 Total unplanned days in hospital at ‘the end of follow up’ (around 18 months): 875 vs. 1476, event rates per month 1.1 (0.8-1.4) vs. 2.7 (1.6-3.7), P = 0.04 Regression analysis showed that assignment to intervention group was a borderline, independent predictor of survival, P = 0.046, Cox proportional hazards model |
Comment on statistical analyses: A rationale for the sample size was provided and the sample size appears to have been adequate. The primary end-point is unusual and does not correspond to any well known statistical test. The statistical tests used to analyse multiple events are unclear and it is not certain which test they used to analyse their primary endpoint. Other points: Not clear how re-admission was determined to be unplanned or planned. Frequency distribution of unplanned re-admissions in the two groups suggests that the difference in unplanned admissions was predominantly amongst those relatively few patients who had three or more admissions in the 6 month follow up period - most of these were in the control group. Intervention group patients accumulated more elective days in hospital (87 vs. 25 P = 0.13) the majority for surgical procedures delayed whilst patient clinically unstable. However, similar proportions of unplanned re-admissions associated with a primary diagnosis of heart failure in each group: 34 (50%) intervention vs. 58 (49%) controls. 88/100 intervention patients received intervention 2 died and 10 withdrew after initial consent. After initial home visit immediate review by primary care physician or cardiologist was requested for 33 patients, 42 intervention patients had a flexible diuretic regimen introduced, 19 patients had greater pharmacy contact arranged and 23 patients had new or increased home support services arranged |
| Jaarsma 2000 | (All reported as intervention gp. vs. control gp.) Please note: The authors of this study make adjustments to their findings both for attrition and multiple testing. For clarity mean scores, SDs and P values for the different scales are not shown in this table unless the authors have stated by that the findings are significant. Measures of QOL Heart Failure Functional Status Inventory: difference NS at 3 months and at 9 months Symptom occurrence: difference NS at 1, 3 and 9 months Change in symptom severity and distress from baseline: considerable attrition at both 3 and 9 months (e.g. for symptom severity only 26/58 responses in intervention group, 42/74 in control group at 9 months), differences NS at 3 months and NS at 9 months after attempt to adjust for attrition by attributing change score of zero to missing values. Psychosocial Adjustment to Illness Scale: differences NS at 3 and 9 months. Cantril’s Ladder of Life: Patients often stated that they had difficulty with this scale resulting in several missing values, differences NS at 1 month (results for 3 and 9 months not given because patients had such difficulty using the scale*). Measures of self-agency and self care behaviour Self care abilities, Appraisal of Self-Care Agency Scale: differences NS at baseline, 3 and 9 months follow up. Self care behaviours: difference NS at baseline and 9 months follow up. At 1 month: 13.8 (SD 3.4) vs. 12. 2 (2.9) P < 0.001, at 3 months: 11.6 (SD 3.1) vs. 10.2 (3.3) P < 0.005. Health care resource use Hospital readmissions (on all patients i.e. 84 intervention and 95 control patients), average days in hospital per patient at 9 months follow up: 9 vs. 9, NS, P value and test not given. Patients with at least one hospital re-admission at 9 months follow up: 31 (37%) vs. 47 (50%), P = 0.06, Chi squared test Cardiac readmissions at 9 months follow up, mean days per patient: 5.1 days (SD 11) vs. 7.1 days (SD 15), NS, P value and test not given Patients with at least one cardiac hospital re-admission at 9 months follow up: 24 (29%) vs. 37 (39%), P = 0. 1, Chi squared test Patients with at least one cardiac hospital re-admission at 9 months follow up: 24 (29%) vs. 37 (39%), P = 0. 1, Chi squared test Patients with at least one hospital re-admission at 1 month follow up: no significant differences between the two groups Hospital readmissions, average days in hospital per patient at 3 months follow up: 5.1 vs. 5.1, NS, P value and test not given. Patients with at least one hospital re-admission at 3 months follow up: 22 (26%) vs. 29 (31%), NS, P value and test not given. Cardiac readmissions at 3 months follow up, mean days per patient: 18 (21 %) vs. 23 (24%) NS, P value and test not given. Hospital readmissions, average days in hospital for cardiac readmission per patient at 3 months follow up: 3. 0 vs. 4.1, NS, P value and test not given. Also reported: Deaths during 9 months follow up : 22 vs. 16 NS |
Comment on statistical analyses: Rationale for sample size given. The exact statistical tests used in the final analysis were unclear. Other points: 186 patients were enrolled in the study, 7 died before discharge from the index admission, by 9 months 47 (26%) of the remaining 179 had died or dropped out, the data for those who remained had a large number of missing values. Differences in self care behaviour scores significantly better at 1 and 3 months in intervention group but mean differences very small and clinical significance unclear |
| Blue 2001 | (All reported as intervention gp. vs. control gp.) Primary endpoint at around 12 months: Number of patients with death from all causes or hospital admission for heart failure: 31 vs. 43, hazard ratio = 0.61 (95% CI 0.38 to 0.96), P = 0.03 Secondary endpoints at around 12 months: Death: 25 v. 25 NS Number of patients with death from all causes or all cause hospital re-admission: 52 vs. 61, hazard ratio = 0. 72 (95% CI 0.49 to 1.40) NS, P = 0.075 Number of patients with hospital re-admission (all causes): 47 vs. 49, P = 0.27, NS Number of patients with hospital re-admission for worsening HF: 12 vs. 26, P = 0.004 Also looked at (at around 12 months): Number of admissions per patient per month (all causes): 0.124 vs. 0.174, hazard ratio = 0.71 (95% CI 0.54 to 0.94) P = 0.018 Number of admissions per patient per month (worsening HF): 0.027 vs. 0.069, hazard ratio = 0.40 (95% CI 0.23 to 0.71) P = 0.0004 Mean days spent in hospital (all causes): 10.3 (SD 19. 0) vs. 16.7 (24.1), P = 0.08 Mean days spent in hospital (worsening HF): 3.43 (12. 2) vs. 7.46 (16.6), P = 0.005 |
Comment on statistical analyses: The size of the study was only just adequate for statistical power based on a calculation for 12 months follow up. Some of the statistical results are presented in an ambiguous way. The study might have benefited from a further exploration of the data with some sensitivity analyses. For example, the primary endpoint includes deaths that occurred in hospital after randomisation. As it happens there were more deaths in the control group than in the intervention group (6 vs. 1), if the analysis is re-done excluding these in pre-discharge hospital deaths the primary endpoint is no longer significant |
| Capomolla 2002 | (All reported as intervention group vs. control group.) Primary outcomes: Relative risk of cardiac death or urgent heart transplantation: RR0.17, favouring intervention, (95% CI 0.06, 0.66) Deaths from cardiac causes: 3/112 (3%) vs. 21/122 (17%), P 0.0007 Total number of hospital readmissions at mean 12 (SD3) months follow up: 13 vs. 78, P<0.00001 (NB not clear if these readmissions are because of haemodynamic instability as stated earlier in the paper.) Total number of patients with at least one rehospitalisation during follow up: 8 vs. 35 P <0.05 Secondary outcomes: QOL (time trade off method): 0.72 (SD 0.17) vs. 0. 63 (SD 0.22) P < 0.008. (ie intervention patients were willing to trade 10 years of their present health for 7.2 years of excellent health, whereas control patients were willing to trade 6.3 years of their present health. (NB only change within the two groups reported.) NYHA functional class: only reported as before and after findings and error in table showing the results of NYHA functional class at one year see below. Also looked at: Mean total care management costs (ie group mean read-mission costs and day hospital costs): $167,785 vs. $178,553, no standard deviations or tests given. Cost utility ratio of the two strategies: $2,244 vs. $2, 409 Incremental analyses showed a cost saving of$1,068 for each QALY gained with the intervention. Urgent heart transplant during follow up: 1 vs. 0. |
Comment on statistical analyses: No sample size provided but study appears to have adequate power and the statistical tests employed are appropriate. There is a serious error in Table 3 on page 1263: NYHA classifications are given on 112/112 usual care patients at one year and on 113/112 day hospital patients at one year despite the fact that we are told that cardiac death occurred in 21/122 patients in the community group and 3 patients in the day hospital group. Comment on cost utility analysis: The study is not from a societal perspective as stated since it does not consider all the costs to the patient (i. e. travel, time off work, home help). The information for the cost utility analysis is poorly presented. ‘Total costs’ should be presented with ‘average’ costs since from the averages it is hard to cross check calculations. It would also have been helpful to see the break down of the costs and quantities used, so the source of these ‘totals’ could be known. A cross check of their calculations suggests that they are consistent but the total costs figures do not seem to be consistent with the averages reported. It would also be helpful to know how they calculated the total QALYs per programme to check to what extent they have allowed for the timing of the deaths within the year. Other points: Not stated how deaths from cardiac causes were identified. Not stated how readmissions because of haemodynamic instability were identified. Total number of deaths in each group not given. Not clear if QOL was same at baseline for both groups. Not all the 112 patients in the intervention received all the components of the intervention: 76% received education and physical training; 47% received cardiovascular risk stratification; 45% received tailored therapy; 19% received multidisciplinary intervention. There were 49 ‘open access interventions’ in the intervention group, these included interventions which would have required admission in the control group |
| Doughty 2002 | (All reported as intervention gp. vs. control gp.) Primary endpoints: Event free survival, time to first hospital admissions or all cause death: P = 0.33 (NS), Kaplan-Meier HRQL, Change in MLHFQ total score from baseline to 12 months between the two groups: P = 0.1 (NS) (change in physical score −11.1 vs. −5.8, P = 0.015, change in emotional score −3.3 both groups, P= 0.97, NS) Secondary endpoints: All cause hospital re-admission rates at 12 months: 1.37 re-admissions per patient per year vs. 1.84 (method of calculation not given), rate difference = 0.47 per patient per year (95% CI 0.16, 0.78) . All cause total hospital bed days at 12 months: 1074 vs. 1170 NS, test statistic not given, 12.3 bed days per patient per year vs. 13.9, mean difference in bed days per patient = 1.6 (95% CI 0.51, 2.7) (method not given) Re-admissions for heart failure at 12 months: 36 vs. 65 NS Also reported: All cause deaths at one year: 19 (19%) vs. 24 (25%) Medication: trend (P = 0.052) for intervention group to be on higher ACEI dose at 12 months, no other significant differences Mean time to 1st hospital re-admission: 102 (SD 104) vs. 122 (SD 116) P =0.4, NS. (method not given) Total all cause re-admissions at 12 months: 120 vs. 154 NS, P value not given (method not given) First all cause re-admissions at 12 months: 64 vs. 59 NS, P value not given (method not given) Subsequent all cause re-admissions 56 vs. 95, P = 0.015 (Fishers exact test - test inappropriate) All cause hospital bed days first re-admissions: 546 vs. 444 (no statistical test result given) All cause bed days during subsequent re-admissions: 528 vs. 726 P = 0.0001 (test method not given) First heart failure related re-admissions at 12 months: 21 vs. 23 NS, test statistic not given Subsequent heart failure related re-admissions at 12 months: 15 vs. 42 P <0.05 (Fishers exact test - test inappropriate) Total hospital bed days for heart failure related re-admissions at 12 months: 358 vs. 561 NS, test statistic not given Hospital bed days for first heart failure related re-admission at 12 months: 219 vs. 195 NS, test statistic not given Hospital bed days for subsequent heart failure related re-admission at 12 months: 139 vs. 366 P = 0.0001 (test method not given) |
Comment on statistical analyses: Trial terminated early apparently because inadequate power to detect difference in primary endpoint. An accompanying editorial article states: “The study was actually prematurely stopped. A provisional estimate of 180 patients per group was made but the final sample size was calculated after 100 patients had been followed for 6 months. The event rate was found to be higher than expected but there was no difference between the two groups for the combined primary endpoint of death or re-admission. Projection of the observed effect size suggested that an order of magnitude of more patients would have been required to achieve a result reaching statistical significance, but even this would probably have had little clinical significance. The follow-up of patients already recruited was completed to allow data for total admissions and quality of life to be analysed” (Cunningham 2001). The explanation of the statistical analysis for the analysis of multiple admissions lacks clarity. Some of the data is continuous but since admissions and bed days are likely to be highly skewed the t test, whose use is mentioned in the paper, would be inappropriate. Also the use of Fisher’s exact test to compare subsequent readmissions does not seem appropriate since this test cannot be used to analyse multiple events. Other comments: Baseline values of MLHFQ not given. 60% of the intervention group attended the first group educational session, 40% attended the six month educational session |
| Harrison 2002 | (All reported as intervention group vs. control group.) Primary outcome on 157 patients: MLHFQ total score at baseline, mean (SD): 44.8 (18. 5) vs. 44.6 (19.5), P = 0.9 (NS) MLHFQ total score at 6 weeks, mean (SD): 27.3 (19. 1) vs. 37.5 (20.3), P for difference = 0.002 MLHFQ total score at 12 weeks, mean (SD): 25.8 (19. 4) vs. 38.4 (18.2), P for difference < 0.001 (Also significant improvements in physical dimension at 6 and 12 weeks and in emotional dimension at 6 but not 12 weeks.) Proportion of patients at 6 weeks with at minimally significant difference in total MLHFQ score (5 points) from baseline, worse, same, better: intervention group 3 (4%), 18 (23%), 58 (73%), control group 17 (22%) , 17 (22%), 42 (55%), P = 0.002. Proportion of patients at 12 weeks with at minimally significant difference in total MLHFQ score from baseline worse, same, better: intervention group 6 (8%), 7 (9%), 65 (83%) v. control group (29%), 10 (13%), 44 (58%), P = 0.001 Secondary outcomes: QOL measured with SF-36: SF 36 total scores not given. Data difficult to interpret. Proportion of patients making an emergency room visit (all causes) during the 12 weeks after discharge (on the 157 patients who were followed up): 29% vs. 46%, P= 0.03, Chi squared test. Proportion of patients re-admitted to hospital (all causes) during the 12 weeks after discharge (on the 157 patients who were followed up): 23% vs. 31%, P = 0. 26, NS |
Comment on statistical analyses: Sample size calculation given, sample size appears satisfactory for primary endpoint. Authors mention lack of power to detect a difference in readmissions. Statistical analyses appear appropriate. |
| Kasper 2002 | (All reported as intervention gp. vs. control gp.) Primary endpoint: Total number of CHF hospital admissions plus all cause deaths: 50 in 102 patients vs. 72 in 98 patients, P = 0. 09 (NS) log transformed t test, P = 0.03 Poisson model comparison (see comment below). Secondary endpoints: Deaths at 6 months: 7 in 102 patients vs. 13 in 98 patients P = 0.14 (NS) log-rank test Re-admissions for CHF: 43 admissions in 26 patients vs. 59 admissions in 35 patients, P = 0.09 (NS) log transformed t test, P = 0.03 Poisson model comparison. All cause hospital admissions plus all cause deaths: 84 in 102 patients vs. 109 in 98 patients, P = 0.13 (NS) log transformed t test, P = 0.04 Poisson model comparison. Event free survival (death or re-admission at 6 months) : P for difference = 0.12 (NS) QOL: change in Minnesota Living with Heart Failure Q (MLHFQ) total score at 6/12 from baseline, mean, median −28.3, −28 vs. −15.7, −15, P = 0.001 Wilcoxon test (lower MLHFQ score = better) Functional status: change in Duke Activity Status Score at 6/12 from baseline, 1.1, 1 (mean, median) vs. 0.8, 1 v, P = 0.44 (NS) Wilcoxon test. (Duke Activity Status Index also NS). Process measures at 6 months: Proportion of patients with systolic dysfunction receiving target vasodilators: 74/80 vs. 43/7, P < 0.001 Dietary compliance “good”or “average”: 65/94 vs. 38/ 85, P = 0.002 At goal weight: 47/94 vs. 17/85, P = 0.001. Medication compliance: NS difference. Cost data: Mean costs: $16,182 vs. $8,789 (NS) 75th centile $6,527 vs. 10,898 (NS) |
Comment on statistical analyses: Poisson model analyses suggested by the Oversight, Data and Safety Monitoring Committee before patient enrolment. However the Poisson model does not hold for these data. This is because the results are over dispersed. This means that there are a few patients with higher numbers of readmissions than would be expected using a Poisson model, so if the data are analysed using Poisson model then an incorrectly small SP results. If the results are analysed using a Poisson model where the over dispersion is accounted for then the SP is 0.11, very near the value from the log-rank test of 0.13. Other comments: Cost difference remained NS if three high cost patients all randomised to the intervention group (two transplant patients and one patient who died shortly after the study) were excluded from cost comparison |
| Krumholz 2002 | (All reported as intervention gp. vs. control gp.) Primary outcome: Death or all cause readmission at one year : 25 vs. 36, RR 0.69, 95% CI 0.52, 0.92, P = 0.01 Risk of HF or other CVD readmission, or death (intervention vs. control): HR0.51, 95% CI 0.29, 0.90, P=0. 02) (Cox proportional hazards model adjusted for age; sex; history of HF and admission serum creatinine) Secondary outcomes: Deaths: 9/44 (20%) vs. 13/44 (30%) RR0.69 (95% CI 0.33 to 1.45), P=0.33, NS. Total readmissions in one year: 49 vs. 80, P=0.06, test not given. Total HF readmissions: 22 vs. 42, P=0.07, NS, test not given. Multiple readmissions 12/44 (27%) vs. 21/44 (48%) RR 0.57 (95% CI 0.33 to 0.99) P=0.05. Risk of HF readmission or death (intervention vs. control): HR 0.52, 95% CI 0.28, 0.98, P=0.04 (Cox proportional hazards model adjusted for age; sex; history of HF and admission serum creatinine). Number of patients experiencing HF or other CVD readmission or death: 22/44 (50%) vs. 35/44 (80%). RR 0.63, 95%CI 0.46, 0.86, P=0.004. Number of patients with at least one heart failure read-mission or death: 18/44 (41%) vs. 30/44 (68%) RR 0. 6 95% CI 0.41,0.89, P=0.01. All cause hospital days readmitted, mean (SD): 10.2 (S 16.8) vs. 15.2 (SD 17.5), P=0.09 test not given. HF or other CVD hospital readmission days, mean (SD): 6.3 (SD 9.2) vs. 12.3 (SD 14.3), P=0.03 test not given. HF hospital days readmitted, mean (SD): 4.1 (SD 6.4) vs. 7.6 (12.1), P=0.1 NS, test not given. Costs: All cause readmission costs, mean per patient: $14,420 vs. $21,935, P=0.02, test not given HF or other CVD readmission costs, mean per patient: $8,888 vs. $18,421, P=0.01, test not given HF readmission costs, mean per patient: $5,232 vs. $9, 575, P=0.04, test not given |
Comment on statistical analyses: The sample size was small and no power calculation was supplied. The statistical analysis seems appropriate, although it is not clear which tests were used for multiple admissions. Cost of care package $530. |
| McDonald 2002 | (All reported as intervention gp. vs. control gp.) Primary endpoint: Number of patients with death or re-admission for HF within 12 weeks 4 v. 12, P = 0.04 (Fisher’s exact test) . 95% CI for Odds ratio 0.07-0.84, not clear how OR was generated Also reported: Deaths at 3 months 3 vs. 3, NS Number of patients with a re-admission for HF within 12 weeks 1 v. 9, statistical test not supplied Number of patients with re-admission for HF within 12 weeks 1 v. 11, P = < 0.01 95% CI for odds ratio 0. 01-0.53, not clear how OR was generated Number of patients with death or re-admission for HF within one month (from McDonald 2001) 0/35 v.O/35, NS Clinical condition (NYHA class), LVEF, BP, U&E: all NS difference QOL (not stated how measured): NS Process measures at 3 months: Patient knowledge of HF 16.3 (SD 2.7) vs. 13.1 (SD 2. 2) (presume mean scores out of 20), P = < 0.01 Patient knowledge of diet 8.3 (SD 2.1) vs. 6.6 (SD 1. 9) (presume mean scores out of 10), P = < 0.01 Carer knowledge of HF: NS difference Mean doses of frusemide, digoxin or ACEI: NS difference |
Comment on statistical analyses: No sample size calculation was performed. Other comments: Data on all cause admissions not supplied. There are discrepancies in two tables between the interim paper (McDonald 2001) and the final results paper (McDonald 2002): the number of patients who died in their index admission or were excluded because of co-morbidity compromising their survival went down between the two papers, as did baseline demographics for the number of patients with an idiopathic aetiology for their HF and with previous admissions for HF within one or three months of the index admission. In addition the rate of recruitment appears to be very different in these two reports |
| Riegel 2002 | (All reported as intervention gp. vs. control gp.) Primary endpoint: (See statistical analyses comment) Mean number of hospitalisations with HF per patient at 3 months: 0.17 (SD 0.43) vs. 0.31 (SD 0.64), P=0. 03, analysis of covariance. Mean number of hospitalisations with HF per patient at 6 months: 0.21 (SD 0.5) vs. 0.41 (SD 0.77), P=0.02, analysis of covariance. Other endpoints: (See statistical analyses comment ) Mean number of all cause hospitalisations per patient at 3 months: 0.45 (SD 0.73) vs. 0.61 (SD 0.88) P=0. 25, analysis of covariance. Mean number of all cause hospitalisations per patient at 6 months: 0.62 (SD 0.88) vs. 0.87 (SD 1.1) P=0.11, analysis of covariance. Proportion of patients re-admitted with HF at 3 months: 14.6% vs. 22.8%, P=0.06, multiple logistic regression Proportion of patients re-admitted with HF at 6 months: 17.7% vs. 27.6%, P=0.06, multiple logistic regression Proportion of patients readmitted with all causes at 3 months: 33.8% vs. 41.2%, P=0.40, multiple logistic regression Proportion of patients readmitted with all causes at 6 months: 43.1% vs. 50.0%, P=0.49, multiple logistic regression Mean number of heart failure related hospital bed days at 3 months: 0.9 (2.3) vs. 1.6(3.9), P=0.56 multiple linear regression Mean number of heart failure related hospital bed days at 6 months: 1.2 (3.1) vs. 2.1 (4.6), P = 0.05, multiple linear regression Mean number of all cause hospital bed days at 3 months: 2.6 (5.0) vs. 3.5 (7.2), P = 0.35 multiple linear regression Mean number of all cause hospital bed days at 6 months: 3.5 (6.6) vs. 4.8 (8.3), P = 0.23 multiple linear regression Mean time to rehospitalisation at 6 months: 128.5 (SD 68.6) vs. 115.7 (SD 68.6), P=0.32, Proportion of patients with multiple readmissions at 6 months: 13.1%. vs. 22.8%, P=0.07 Mean emergency department visits at 6 months: 0.14 (SD 0.45) vs. 0.11 (SD 0.94), P=0.58 Patient satisfaction at 6 months: NB available on only 184 of the 242 patients the researchers attempted to survey, number in intervention and control groups not given, 22.88 (SD 2.85) vs. 21.66 (SD 3.44), P=0.01, clinical importance of1.22 point difference in this scale unclear, scale not validated. Also looked at: Cost of acute care at 6 months: $1192(SD 3674) vs. $2186 (SD 6729), P=0.07 Proportion of patients alive at 6 months: 87.7%. vs. 86%, P= not stated |
Comment on statistical analyses: For the primary outcomes and other hospitalisation data the statistical tests used appear to be are inappropriate as the data are not normally distributed. Another major criticism of this paper is that it gives no information about the randomisation clusters: how many there were; how many physicians in each; how many patients in each on average; and how many clusters were finally analysed. This information is necessary to assess generalisability, bias, robustness of analysis and importance of adjusting for clustering. A related point is that if the physicians were matched then if one of a pair refused to participate, the other should also have been omitted and it is not clear that this has been done. The sample size calculation appears to take no account of clustering, which will result in an underestimate of numbers needed. However, the number of patients estimated is about the same as the number of physicians, so it is possible that cluster sizes were very small in which case the sample size calculation would not be much altered. (It is not possible to tell how big cluster sizes are because we are not told how many clusters were lost to follow-up.) Cost of intervention was $443. |
| Laramee 2003 | (All reported as intervention gp. vs. control gp.) Primary endpoint: (results on 131 intervention and 125 control patients) Number of patients with a readmission (all causes) during 90 days after discharge: 49 (37%) vs. 46 (37%), P >0.99, NS Secondary endpoints: Adherence to the treatment plan: intervention group adhered to treatment plan better than usual care group at both 4 and 12 weeks with regard to daily weights, checking for oedema, low salt diet and fluid intake recommendations (however differences were small). Patient satisfaction: Mean scores on patient satisfaction survey were significantly higher in intervention group (however differences were small). Medications at 12 weeks (results on 128 intervention and 113 control patients): Taking ACEIs or ARBs: 108 (84%) vs. 90 (80%), P = 0.40, NS Taking beta blocker: 89 (70%) vs. 70 (62%), P = 0.22, NS Taking target does of ACEI of ARB: 64 (63%) vs. 42 (49%), P = 0.08, NS Target does of beta blocker: 27 (32%) vs. 18 (29%), P = 0.72, NS Also looked at: (results on 131 intervention and 125 control patients) Number of patients with a CHF readmission during 90 days after discharge: 18 (14%) vs. 21 (17%), P = 0.49, NS Number of patients with a cardiac readmission during 90 days after discharge: 15 (11%) vs. 10 (8%), P = 0. 40, NS Number of patients with more than one readmission: 7% vs. 8%, P = 0.83. Mean (SD) LOS in hospital for patients with at least one readmission: 6.9 (6.5) vs. 9.5 (9.8) P = 0.15, NS Median (interquartile range) LOS in hospital for patients with at least one readmission: 5 (2-8) vs. 7 (2-10) P = 0.37, NS. Deaths: (results on 141 intervention and 146 control patients) 13 (9%) vs. 15 (10%), P = 0.84, NS Cost data: (results on 135 intervention and 127 control patients who completed the 12 week study period) Total costs means medians: $23,054 vs. 25,536 P = 0. 39 NS; $15.979 vs. $18,662 P = 0.14 NS Total readmission costs means: $5,253 vs. $5,163 P = 0.96 NS Total outpatient costs means: $ 1,552 vs. C $1,307 P = 0.28 Initial admission costs means: $ 16,119 vs. $19,081 P = 0.18 |
Comment on statistical analyses: No sample size calculation given. Statistical analyses appear appropriate. Other comments: Readmission and LOS outcomes not given on ‘patients whose participation was terminated early and were not known to have been readmitted’. Outcome assessment not masked, possibility of bias in patient satisfaction scores |
| gp = group patient, U&E = urea and electrolyte levels, * information from personal communication with author |
Table 4. Delphi quality criteria table.
| Study | Randomised? | Allocation concealed |
Similar at baseline? |
Eligibility specifd. |
Assessor masked? |
Point estimates etc? |
Intention to treat? |
Notes |
|---|---|---|---|---|---|---|---|---|
| Rich 1993 | Y* | U | Y(1)* | Y | N* | Y* | Y | (1) Intervention patients significantly older. |
| Rich 1995 | Y | Y | Y* | Y | N(2)* | Y | Y | (2) Those collecting the outcome data on admissions were not masked but those collecting data on medication and dietary compliance were masked as were those analysing the data |
| Cline 1998 | Y | U | Y(3) | Y | U | N | U | (3) Mean LVEF significantly lower in intervention group. |
| Ekman 1998 | Y | Y* | Y(4) | Y | N* | Y | Y | (4) Proportion with atrial fibrillation higher in control group |
| Rainville 1999 | Y* | U* | N | Y | N* | N | N* | |
| Stewart 1999 | Y | Y | Y | Y | Y | Y | Y | |
| Jaarsma 2000 | Y* | U* | U(5) | Y | N | N | N | (5) Considerable attrition of study subjects but only those who remained in the study at 9 months are compared at baseline |
| Blue 2001 | Y | Y | Y | Y | Y | Y | U | |
| Capomolla 2002 | U(6) | U | Y | Y(7) | N | Y | U | (6) Method of randomisation not specified (7) apparently no exclusion criteria |
| Doughty 2002 | Y | U | Y | Y | U | N | Y | |
| Harrison 2002 | Y | Y | Y | Y | Y* | Y | N | |
| Kasper 2002 | Y | Y | U(9) | Y | Y | N | Y | (9) Information on presence of all risk factors identified by authors not supplied |
| Krumholz 2002 | Y* | N* | Y(10) | Y | Y | Y | U | (10) Intervention group significantly older with lower incidence of prior CABG and fewer prescribed calcium channel blockers |
| McDonald 2002 | Y* | Y* | Y | Y | N* | N | Y | |
| Riegel 2002 | U(12) | U | Y(13) | Y | U | N | U | (12) Method of randomisation not specified. (13) control patients significantly more likely to have COPD |
| Laramee 2003 | Y(14)* | N* | Y(15) | Y | N | N | N* | (14) Patients chose from sealed envelopes. |
WHAT’S NEW
Last assessed as up-to-date: 31 January 2005.
| Date | Event | Description |
|---|---|---|
| 8 September 2008 | Amended | Converted to new review format. |
HISTORY
Protocol first published: Issue 4, 2000
Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 1 February 2005 | New citation required and conclusions have changed | Substantive amendment |
CONTRIBUTIONS OF AUTHORS
Stephanie Taylor:
Conceiving the review
Designing the review
Coordinating the review
Data collection for the review
Developing search strategy
Undertaking searches
Screening search results
Organising retrieval of papers
Screening retrieved papers against inclusion criteria
Appraising quality of papers
Abstracting data from papers
Writing to authors of papers for additional information
Obtaining and screening data on unpublished studies
Data management for the review
Entering data into RevMan
Analysis of data
Interpretation of data
Providing methodological perspective
Providing a clinical perspective
Writing the review
Martin Underwood:
Conceiving the review
Designing the review
Coordinating the review
Data collection for the review
Developing search strategy
Undertaking searches
Screening search results
Organising retrieval of papers
Screening retrieved papers against inclusion criteria
Obtaining and screening data on unpublished studies
Analysis of data
Interpretation of data
Providing general advice on the review
Maggie Falshaw:
Conceiving the review
Designing the review
Coordinating the review
Data collection for the review
Developing search strategy
Undertaking searches
Screening search results
Organising retrieval of papers
Screening retrieved papers against inclusion criteria
Analysis of data
Interpretation of data
Providing general advice on the review
Suzanne Parsons:
Conceiving the review
Designing the review
Coordinating the review
Data collection for the review
Developing search strategy
Undertaking searches
Screening search results
Organising retrieval of papers
Screening retrieved papers against inclusion criteria
Analysis of data
Interpretation of data
Providing general advice on the review
Sonja Hood:
Conceiving the review
Designing the review
Coordinating the review
Data collection for the review
Developing search strategy
Undertaking searches
Screening search results
Organising retrieval of papers
Screening retrieved papers against inclusion criteria
Analysis of data
Interpretation of data
Providing general advice on the review
Janine Bestall:
Appraising quality of papers
Abstracting data from papers
Analysis of data
Interpretation of data
Providing general advice on the review
Sarah Cotter:
Abstracting data from papers
Analysis of data
Providing methodological perspective
Lesley Wood:
Abstracting data from papers
Analysis of data
Providing methodological perspective
Footnotes
DECLARATIONS OF INTEREST
None known
References to studies included in this review
* Indicates the major publication for the study
- Blue 2001 {published data only} .*; Blue L, Lang E, McMurray JJ, Davie AP, McDonagh TA, Murdoch DR, et al. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ. 2001;323:715–8. doi: 10.1136/bmj.323.7315.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue L, McMurray JJV. A specialist nurse-led, home-based intervention in Scotland. In: Stewart S, Blue L, editors. Improving outcomes in chronic heart failure: a practical guide to specialist nurse intervention. BMJ Books; 2001. [Google Scholar]
- Capomolla 2002 {published data only} .*; Capomolla S, Febo O, Ceresa M, Caporotondi A, Guazzotti G, La Rovere M, et al. Cost/utility ratio in chronic heart failure: comparison between heart failure management program delivered by day-hospital and usual care. Journal of the American College of Cardiology. 2002;40:1259–66. doi: 10.1016/s0735-1097(02)02140-x. [DOI] [PubMed] [Google Scholar]
- Cline 1998 {published data only} .Cline C, Iwarson A. Nurse-led clinics for the management of heart failure in Sweden. In: Stewart S, Blue L, editors. Improving outcomes in chronic heart failure: a practical guide to specialist nurse intervention. BMJ Books; 2001. [Google Scholar]
- *; Cline CM, Israelsson BY, Willenheimer RB, Broms K, Erhardt LR. Cost effective management programme for heart failure reduces hospitalisation. Heart. 1998;80:442–6. doi: 10.1136/hrt.80.5.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty 2002 {published data only} .*; Doughty RN, Wright SP, Pearl A, Walsh HJ, Muncaster S, Whalley GA. Randomized, controlled trial of integrated heart failure management. The Auckland Heart Failure Management Study. European Heart Journal. 2002;23:139–46. doi: 10.1053/euhj.2001.2712. [DOI] [PubMed] [Google Scholar]
- Doughty RN, Wright SP, Walsh HJ, Muncaster S, Pearl A, Sharpe N. Integrated care for patients with chronic heart failure: the New Zealand experience. In: Stewart S, Blue L, editors. Improving outcomes in chronic heart failure: a practical guide to specialist nurse intervention. BMJ Books; 2001. [Google Scholar]
- Ekman 1998 {published data only} .*; Ekman I, Andersson B, Ehnfors M, Matejka G, Persson B, Fagerberg B. Feasibility of a nurse-monitored, outpatient-care programme for elderly patients with moderate-to-severe, chronic heart failure. European Heart Journal. 1998;19:1254–60. doi: 10.1053/euhj.1998.1095. [DOI] [PubMed] [Google Scholar]
- Ekman I, Andersson B, Ehnfors M, Matejka G, Persson B, Fagerberg B. Outpatient care programmes for the elderly [letter] European Heart Journal. 1999;20:393–4. [PubMed] [Google Scholar]
- Harrison 2002 {published data only} .Harrison MB, Browne GB, Roberts J, Tugwell P, Gafni A, Graham ID. Quality of life of individuals with heart failure: a randomized trial of the effectiveness of two models of hospital-to-home transition. Medical Care. 2002;40:271–82. doi: 10.1097/00005650-200204000-00003. [DOI] [PubMed] [Google Scholar]
- Jaarsma 2000 {published data only} .*; Jaarsma T, Halfens R, Tan F, Abu-Saad HH, Dracup K, Diederiks J. Self-care and quality of life in patients with advanced heart failure: the effect of a supportive educational intervention. Heart & Lung: Journal of Acute & Critical Care. 2000;29:319–30. doi: 10.1067/mhl.2000.108323. [DOI] [PubMed] [Google Scholar]
- Jaarsma T, Halfens R, Tan F, Abu-Saad HH, Dracup K, Gorgels T, et al. Effects of education and support on self-care and resource utilization in patients with heart failure. European Heart Journal. 1999;20:673–82. doi: 10.1053/euhj.1998.1341. [DOI] [PubMed] [Google Scholar]
- Kasper 2002 {published data only} .*; Kasper EK, Gerstenblith G, Hefter G, Van Anden E, Brinker JA, Thiemann DR, et al. A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital re-admission. Journal of the American College of Cardiology. 2002;39:471–80. doi: 10.1016/s0735-1097(01)01761-2. [DOI] [PubMed] [Google Scholar]
- Krumholz 2002 {published data only} .*; Krumholz HM, Amatruda J, Smith GL, Mattera JA, Roumanis SA, Radford MJ, et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. Journal of the American College of Cardiology. 2002;39:83–9. doi: 10.1016/s0735-1097(01)01699-0. [DOI] [PubMed] [Google Scholar]
- Laramee 2003 {published data only} .*; Laramee AS, Levinsky SK, Sargent J, Ross R, Callas P. Case management in a heterogeneous congestive heart failure population: A randomized controlled trial. Archives of Internal Medicine. 2003;163:809–17. doi: 10.1001/archinte.163.7.809. [DOI] [PubMed] [Google Scholar]
- McDonald 2002 {published data only} .McDonald K, Ledwidge M, Cahill J, Quigley P, Maurer B, Travers B, et al. Elimination of early rehospitalization in a randomized, controlled trial of multidisciplinary care in a high-risk, elderly heart failure population: the potential contributions of specialist care, clinical stability and optimal angiotensin-converting enzyme inhibitor dose at discharge. European Journal of Heart Failure. 2000;3:209–15. doi: 10.1016/s1388-9842(00)00134-3. [DOI] [PubMed] [Google Scholar]
- *; McDonald K, Ledwidge M, Cahill J, Quigley P, Maurer B, Travers B, et al. Heart failure management: Multidisciplinary care has intrinsic benefit above the optimization of medical care. Journal of Cardiac Failure. 2002;8:142–8. doi: 10.1054/jcaf.2002.124340. [DOI] [PubMed] [Google Scholar]
- Rainville 1999 {published data only} .Rainville EC. Impact of pharmacist interventions on hospital re-admissions for heart failure. American Journal of Health-System Pharmacy. 1999;56:1339–42. [PubMed] [Google Scholar]
- Rich 1993 {published data only} .*; Rich MW, Vinson JM, Sperry JC, Shah AS, Spinner LR, Chung MK, et al. Prevention of re-admission in elderly patients with congestive heart failure: Results of a prospective, randomized pilot study. Journal of General Internal Medicine. 1993;8:585–9. doi: 10.1007/BF02599709. [DOI] [PubMed] [Google Scholar]
- Rich 1995 {published data only} .*; Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the re-admission of elderly patients with congestive heart failure. New England Journal of Medicine. 1995;333:1190–5. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- Rich MW, Gray DB, Beckham V, Wittenberg C, Luther P. Effect of a multidisciplinary intervention on medication compliance in elderly patients with congestive heart failure. American Journal of Medicine. 1996;101:270–6. doi: 10.1016/s0002-9343(96)00172-6. [DOI] [PubMed] [Google Scholar]
- Riegel 2002 {published data only} .Riegel B, Carlson B, Glaser D, Kopp Z, Romero TE. Standardized telephonic case management in a Hispanic heart failure population: An effective intervention. Disease Management & Health Outcomes. 2002;10:241–9. [Google Scholar]
- *; Riegel B, Carlson B, Kopp Z, LePetri B, Glaser D, Unger A. Effect of a standardized nurse case-management telephone intervention on resource use in patients with chronic heart failure. Archives of Internal Medicine. 2002;162:705–12. doi: 10.1001/archinte.162.6.705. [DOI] [PubMed] [Google Scholar]
- Stewart 1999a {published data only} .Stewart S, Horowitz JD. A specialist nurse-led intervention in Australia. In: Stewart S, Blue L, editors. Improving outcomes in chronic heart failure: a practical guide to specialist nurse intervention. BMJ Books; 2001. [Google Scholar]
- *; Stewart S, Marley JE, Horowitz JD. Effects of a multidisciplinary, home-based intervention on unplanned re-admissions and survival among patients with chronic congestive heart failure: a randomised controlled study. Lancet. 1999;354:1077–83. doi: 10.1016/s0140-6736(99)03428-5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Akosah 2002 {published data only} .*; Akosah KO, Schaper AM, Havlik P, Barnhart S, Devine S. Improving care for patients with chronic heart failure in the community: The importance of a disease management program. Chest. 2002;122:906–12. doi: 10.1378/chest.122.3.906. [DOI] [PubMed] [Google Scholar]
- Azevedo 2002 {published data only} .*; Azevedo A, Pimenta J, Dias P, Bettencourt P, Ferreira A, Cerqueira-Gomes M. Effect of a heart failure clinic on survival and hospital readmission in patients discharged from acute hospital care. European Journal of Heart Failure. 2002;4:353–9. doi: 10.1016/s1388-9842(02)00013-2. [DOI] [PubMed] [Google Scholar]
- Barth 2001 {published data only} .*; Barth V. A nurse-managed discharge program for congestive heart failure patients: outcomes and costs. Home Health Care Management & Practice. 2001;13:436–43. [Google Scholar]
- Benatar 2003 {published data only} .*; Benatar D, Bondmass M, Ghitelman J, Avitall B. Outcomes of chronic heart failure. Archives of Internal Medicine. 2003;163(3):347–52. doi: 10.1001/archinte.163.3.347. : 15534. [DOI] [PubMed] [Google Scholar]
- Cordisco 1999 {published data only} .*; Cordisco ME, Benjaminovitz A, Hammond K, Mancini D. Use of telemonitoring to decrease the rate of hospitalization in patients with severe congestive heart failure. American Journal of Cardiology. 1999;84:860–2. doi: 10.1016/s0002-9149(99)00452-x. [DOI] [PubMed] [Google Scholar]
- Costantini 2001 {published data only} .*; Costantini O, Huck K, Carlson MD, Boyd K, Buchter CM, Raiz P. Impact of a guideline-based disease management team on outcomes of hospitalized patients with congestive heart failure. Archives of Internal Medicine. 2001;161:177–82. doi: 10.1001/archinte.161.2.177. : 5712. [DOI] [PubMed] [Google Scholar]
- de Lusignan 1999 {published data only} .*; de Lusignan S, Meredith K, Wells S, Leatham E, Johnson P. A controlled pilot study in the use of telemedicine in the community on the management of heart failure-a report of the first three months. Studies in Health Technology & Informatics. 1999;64:126–37. [PubMed] [Google Scholar]
- Evans 1993 {published data only} .Evans RL, Hendricks RD. Evaluating hospital discharge planning: a randomized clinical trial. Medical Care. 1993;31:358–70. doi: 10.1097/00005650-199304000-00007. [DOI] [PubMed] [Google Scholar]
- Farag 1967 {published data only} .*; Farag SA, Mozar HN. Preventing recurrences of congestive heart failure. Journal of The American Dietetic Association. 1967;51:26–8. [PubMed] [Google Scholar]
- Fitzgerald 1994 {published data only} .Fitzgerald JF, Smith DM, Martin DK, Freedman JA, Katz BP. A case manager intervention to reduce readmissions. Archives of Internal Medicine. 1994;154:1721–9. [PubMed] [Google Scholar]
- Gattis 1999 {published data only} .*; Gattis WA, Hasselblad V, Whellan DJ, O’Connor CM. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team: results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) Study. Archives of Internal Medicine. 1999;159:1939–45. doi: 10.1001/archinte.159.16.1939. [DOI] [PubMed] [Google Scholar]
- Goodyer 1995 {published data only} .*; Goodyer LI, Miskelly F, Milligan P. Does encouraging good compliance improve patients’ clinical condition in heart failure? British Journal of Clinical Practice. 1995;49:173–6. [PubMed] [Google Scholar]
- Grancelli 2003 {published data only} .Grancelli H, Varini S, Ferrante D, Schwartzman R, Zambrano C, Soifer S, et al. Randomized controlled trial of telephone intervention in chronic heart failure (DIAL): study design and preliminary observations. Journal of Cardiac Failure. 2003;9:172–9. doi: 10.1054/jcaf.2003.33. [DOI] [PubMed] [Google Scholar]
- Hanchett 1967 {published data only} .*; Hanchett E, Torrens PR. A public health home nursing program for outpatients with heart diseases. Public Health Reports. 1967;82:683–8. [PMC free article] [PubMed] [Google Scholar]
- Hansen 1992 {published data only} .Hansen FR, Spedtsberg K, Schroll M. Geriatric follow-up by home visits after discharge from hospital: a randomized controlled trial. Age & Ageing. 1992;21:445–50. doi: 10.1093/ageing/21.6.445. [DOI] [PubMed] [Google Scholar]
- Heidenreich 1999 {published data only} .*; Heidenreich PA, Ruggerio CM, Massie BM. Effect of a home monitoring system on hospitalization and resource use for patients with heart failure. American Heart Journal. 1999;138:633–40. doi: 10.1016/s0002-8703(99)70176-6. [DOI] [PubMed] [Google Scholar]
- Hughes 2000 {published data only} .Hughes SL, Weaver FM, Giobbie-Hurder A, Manheim L, Henderson W, Kubal JD, et al. Effectiveness of team-managed home-based primary care: A randomized multicenter trial. Journal of the American Medical Association. 2000;284:2877–85. doi: 10.1001/jama.284.22.2877. [DOI] [PubMed] [Google Scholar]
- Jerant 2001 {published data only} .*; Jerant AF, Azari R, Nesbitt TS. Reducing the cost of frequent hospital admissions for congestive heart failure: a randomized trial of a home telecare intervention. Medical Care. 2001;39:1234–45. doi: 10.1097/00005650-200111000-00010. [DOI] [PubMed] [Google Scholar]
- Johnson 2000 {published data only} .*; Johnson B, Wheeler L, Deuser J, Sousa KH. Outcomes of the Kaiser Permanente Tele-Home Health Research Project. Archives of Family Medicine. 2000;9:40–5. doi: 10.1001/archfami.9.1.40. [DOI] [PubMed] [Google Scholar]
- Lin 2001 {published data only} .*; Lin Y-P, Tsai Y-F. Effects of systematic nursing interventions on knowledge, attitude and self-care behaviors of patients with heart failure. Tzu Chi Medical Journal. 2001;120:999–1006. [Google Scholar]
- Naylor 1994 {published data only} .*; Naylor M, Brooten D, Jones R, Lavizzo-Mourey R, Mezey M, Pauly M. Comprehensive discharge planning for the hospitalized elderly. A randomized clinical trial. Annals of Internal Medicine. 1994;120:999–1006. doi: 10.7326/0003-4819-120-12-199406150-00005. [DOI] [PubMed] [Google Scholar]
- Naylor 1999 {published data only} .*; Naylor MD, Brooten D, Campbell R, Jacobsen BS, Mezey MD, Pauly MV, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281:613–20. doi: 10.1001/jama.281.7.613. [DOI] [PubMed] [Google Scholar]
- Naylor MD, McCauley KM. The effects of a discharge planning and home follow-up intervention on elders hospitalized with common medical and surgical cardiac conditions. Journal of Cardiovascular Nursing. 1999;14:44–54. doi: 10.1097/00005082-199910000-00006. [DOI] [PubMed] [Google Scholar]
- Philbin 2000 {published data only} .*; Philbin EF, Rocco TA, Jr, Lindenmuth NW, Ulrich K, McCall M, Jenkins PL. The results of a randomized trial of a quality improvement intervention in the care of patients with heart failure. American Journal of Medicine. 2000;109:443–9. doi: 10.1016/s0002-9343(00)00544-1. [DOI] [PubMed] [Google Scholar]
- Riegel 2000 {published data only} .*; Riegel B, Carlson B, Glaser D, Hoagland P. Which patents with heart failure respond best to multidisciplinary disease management? Journal of Cardiac Failure. 2000;6:290–9. doi: 10.1054/jcaf.2000.19226. [DOI] [PubMed] [Google Scholar]
- Rubin 1992 {published data only} .Rubin CD, Sizemore MT, Loftis PA, Adams-Huet B, Anderson RJ. The effect of geriatric evaluation and management on Medicare reimbursement in a large public hospital: a randomized clinical trial. Journal of the American Geriatrics Society. 1992;40:989–95. doi: 10.1111/j.1532-5415.1992.tb04474.x. [DOI] [PubMed] [Google Scholar]
- Schneider 1993 {published data only} .*; Schneider JK, Hornberger S, Booker J, Davis A, Kralicek R. A medication discharge planning program: measuring the effect on readmissions. Clinical Nursing Research. 1993;2:41–53. doi: 10.1177/105477389300200105. [DOI] [PubMed] [Google Scholar]
- Serxner 1998 {published data only} .Serxner S, Miyaji M, Jeffords J. Congestive heart failure disease management study: a patient education intervention. Congestive Heart Failure. 1998;4:23–8. [Google Scholar]
- Stewart 1998a {published data only} .*; Stewart S, Pearson S, Luke CG, Horowitz JD. Effects of home-based intervention on unplanned re-admissions and out-of-hospital deaths. Journal of the American Geriatrics Society. 1998;46:174–80. doi: 10.1111/j.1532-5415.1998.tb02535.x. [DOI] [PubMed] [Google Scholar]
- Stewart 1998b {published data only} .*; Stewart S, Pearson S, Horowitz JD. Effects of a home-based intervention among patients with congestive heart failure discharged from acute hospital care. Archives of Internal Medicine. 1998;158:1067–72. doi: 10.1001/archinte.158.10.1067. [DOI] [PubMed] [Google Scholar]
- Stewart 1999b {published data only} .*; Stewart S, Vandenbroek AJ, Pearson S, Horowitz JD. Prolonged beneficial effects of a home-based intervention on unplanned re-admissions and mortality among patients with congestive heart failure. Archives of Internal Medicine. 1999;159:257–61. doi: 10.1001/archinte.159.3.257. [DOI] [PubMed] [Google Scholar]
- Stewart 2002b {published data only} .*; Stewart S, Horowitz JD. Home-based intervention in congestive heart failure: long-term implications on readmission and survival. Circulation. 2002;105:2861–6. doi: 10.1161/01.cir.0000019067.99013.67. [DOI] [PubMed] [Google Scholar]
- Topp 1998 {published data only} .Topp R, Tucker D, Weber C. Effect of a clinical case manager/clinical nurse specialist on patients hospitalized with congestive heart failure. Nursing Case Management. 1998;3:140–5. [PubMed] [Google Scholar]
- Townend 1988 {published data only} .*; Townsend J, Piper M, Frank AO, Dyer S, North WR, Meade TW. Reduction in hospital readmission stay of elderly patients by a community based hospital discharge scheme: a randomised controlled trial. BMJ. 1988;297:544–7. doi: 10.1136/bmj.297.6647.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum 1993 {published data only} .van Rossum E, Frederiks CM, Philipsen H, Portengen K, Wiskerke J, Knipschild P. Effects of preventive home visits to elderly people. BMJ. 1993;307:27–32. doi: 10.1136/bmj.307.6895.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger 1996 {published data only} .Weinberger M, Oddone EZ, Henderson WG. Does increased access to primary care reduce hospital re-admissions? New England Journal of Medicine. 1996;334:1441–7. doi: 10.1056/NEJM199605303342206. [DOI] [PubMed] [Google Scholar]
- Williams 1994 {published data only} .Williams H, Blue B, Langlois PF. Do follow-up home visits by military nurses of chronically ill medical patients reduce readmissions? Military Medicine. 1994;159:141–4. [PubMed] [Google Scholar]
References to studies awaiting assessment
- Goldberg 2003 {published data only} .Goldberg LR, Piette JD, Walsh MN, Frank TA, Jaski BE, Smith AL, et al. Randomized trial of a daily electronic home monitoring system in patients with advanced heart failure: The Weight Monitoring in Heart Failure (WHARF) trial. American Heart Journal. 2003;146:705–12. doi: 10.1016/S0002-8703(03)00393-4. [DOI] [PubMed] [Google Scholar]
- Ledwidge {published data only} .Ledwidge M, Barry M, Cahill J, et al. Is multidisciplinary care of heart failure cost-beneficial when combined with optimal medical care? Eur J Heart Fail. 2003;5:381–9. doi: 10.1016/s1388-9842(02)00235-0. [DOI] [PubMed] [Google Scholar]
- REACT {published data only} .Tsuyuki RT, Fradette M, Johnson JA, et al. Multicentre disease management program for hospitalized patients with heart failure: the Review of Education on ACE inhibitors in Congestive heart failure Treatment (REACT) Study. J Card Fail. 2004;10(6):473–80. doi: 10.1016/j.cardfail.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Stromberg {published data only} .Stromberg A, Martensson J, Fridlund B, et al. Nurse-led heart failure clinics improve survival and self-care behaviour in patients with heart failure. Eur Heart J. 2003;24:1014–23. doi: 10.1016/s0195-668x(03)00112-x. [DOI] [PubMed] [Google Scholar]
- Trochu {published and unpublished data} .Trochu JN, Baleynaud S, Mialet G, et al. Efficacy of a multidisciplinary management of chronic heart failure patients: one year results of a multicentre randomized trial in French medical practice. Eur Heart J. 2004 In press. [Google Scholar]
- Woodend {published data only}
References to ongoing studies
- Hardman S {published data only} .The evaluation of a nurse-led intervention to improve self-management for patients admitted to hospital with a diagnosis of heart failure (due to left ventricular systolic dysfunction).. Ongoing study NA, study likely to be completed in 2005..
- Massie 2001 {published data only} .A controlled trial of heart failure management programs. Ongoing study NA.
- Moser 2000 {published data only} .Community case management decreases rehospitalisation rates and costs and improves quality of life in heart failure patients with preserved and non-preserved left ventricular function: a randomised controlled trial. Ongoing study NA.
- Pugh 1999 {published data only} .*; Pugh LC, Tringali RA, Boehmer J, Blaha C, Kruger NR, Capauna TA, et al. Partners in care: a model of collaboration. Holistic Nursing Practice. 1999;13:61–5. doi: 10.1097/00004650-199901000-00010. [DOI] [PubMed] [Google Scholar]
- TEN-HMS {published data only} .Colleta AP, Louis AA, Clark AL, Nitkin N, Cleland JGF. Clinical trials update from the European Society of Cardiology: CARMEN, EARTH, OPTIMAAL, ACE, TEN-HMS, MAGIC, SOLVD-X and PATH-CHF II. European Journal of Heart Failure. 2002;4:661–6. doi: 10.1016/s1388-9842(02)00176-9. [DOI] [PubMed] [Google Scholar]
- Thompson{unpublished data only} .Thompson DR, Roebuck A, Stewart S. Effects of a nurse-led, clinic and home-based intervention on recurrent hospital use in chronic heart failure (in press) European Journal of Heart Failure. 2005 doi: 10.1016/j.ejheart.2004.10.008. [DOI] [PubMed] [Google Scholar]
Additional references
- AHA 2004 .American Heart Association [Accessed 25/01/2004];Heart disease and stroke statistics - 2004 update. www.americanheart.org/downloadable/heart/1072969766940HSStats2004Update.pdf.
- Clarke 1994 .Clarke KW, Gray D, Hampton JR. Evidence of inadequate investigation and treatment of patients with heart failure. British Heart Journal. 1994;71:584–7. doi: 10.1136/hrt.71.6.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland 1999 .Cleland JGF, Gemmell I, Khand A, Boddy A. Is the prognosis of heart failure improving? European Journal of Heart Failure. 1999;1:229–41. doi: 10.1016/s1388-9842(99)00032-x. [DOI] [PubMed] [Google Scholar]
- Cleland 2003 .Cleland JGF, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, et al. The EuroHeart Failure survey programme - a survey on the quality of care among patients with heart failure in Europe. European Heart Journal. 2003;24:442–63. doi: 10.1016/s0195-668x(02)00823-0. [DOI] [PubMed] [Google Scholar]
- Cowie 1997 .Cowie MR, Mosterd A, Wood DA, Deckers JW, et al. The epidemiology of heart failure. European Heart Journal. 1997;18:208–25. doi: 10.1093/oxfordjournals.eurheartj.a015223. [DOI] [PubMed] [Google Scholar]
- Cowie 1999 .Cowie MR. Annotated references in epidemiology. European Journal of Heart Failure. 1999;1:101–7. doi: 10.1016/S1388-9842(98)00008-7. [DOI] [PubMed] [Google Scholar]
- Cowie 2000 .Cowie MR, Wood DA, Coats AS, Thompson SG, Suresh V, Poole-Wilson PA, et al. Survival of patients with a new diagnosis of heart failure: a population based study. Heart. 2000;83:505–10. doi: 10.1136/heart.83.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson 1995 .Eriksson H. Heart failure: a growing public health problem. Journal of Internal Medicine. 1995;237:135–41. doi: 10.1111/j.1365-2796.1995.tb01153.x. [DOI] [PubMed] [Google Scholar]
- Feenstra 1998 .Feenstra J, Grobbee DE, Jonkman FAM, et al. Prevention of relapse in patients with congestive heart failure: the role of precipitating factors. Heart. 1998;80:432–6. doi: 10.1136/hrt.80.5.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havranek 2002 .Havranek EP, Masoudi FA, Westfall KA, Wolfe P, Ordin DL, Krumholz HM. Spectrum of heart failure in older patients: results from the National Heart Failure project. American Heart Journal. 2002;143:412–7. doi: 10.1067/mhj.2002.120773. [DOI] [PubMed] [Google Scholar]
- Jong 2002 .Jong P, Vowinckel E, Liu PP, Gong Y, Tu JV. Prognosis and determinants of survival in patients newly hospitalized for heart failure: a population-based study. Archives of Internal Medicine. 2002;162:1689–94. doi: 10.1001/archinte.162.15.1689. [DOI] [PubMed] [Google Scholar]
- Kannel 1991 .Kannel WB, Belanger AJ. Epidemiology of heart failure. American Heart Journal. 1991;121:951–7. doi: 10.1016/0002-8703(91)90225-7. [DOI] [PubMed] [Google Scholar]
- Krumholz 1997 .Krumholz HM, Parent EM, Tu N, Vaccarino V, Wang Y, Radford MJ, et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Archives of Internal Medicine. 1997;157:99–104. [PubMed] [Google Scholar]
- Levy 2002 .Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KKL, et al. Long term trends in incidence and survival with heart failure. New England Journal of Medicine. 2002;347:1397–402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- McMurray 1993 .McMurray J, McDonagh T, Morrison CE, et al. Trends in hospitalization rates for heart failure in Scotland 1980-1990. European Heart Journal. 1993;14:1158–63. doi: 10.1093/eurheartj/14.9.1158. [DOI] [PubMed] [Google Scholar]
- Michalsen 1998 .Michalsen A, Konig G, Thimme W. Preventable causative factors leading to hospital admission with decompemsated heart failure. Heart. 1998;80:437–41. doi: 10.1136/hrt.80.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE 2003 .National Institute for Clinical effectiveness (NICE) Chronic heart failure. NICE; 2003. Clinical Guideline 5. [Google Scholar]
- Riegel 2001 .Riegel B, LePetri R. Heart failure disease management models. In: Moser DK, Riegel B, editors. Improving outcome in heart failure an interdisciplinary approach. Aspen; Maryland: 2001. pp. 267–81. [Google Scholar]
- Stewart 1989 .Stewart AL, Greenfiled S, Hays RD, et al. Functional status and well-being of patients with chronic conditions. JAMA. 1989;262:907–13. [PubMed] [Google Scholar]
- Stewart 2001b .Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJV. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. European Journal of Heart Failure. 2001;3:315–22. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- Stewart 2002a .Stewart S, Jenkins A, Buchan S, McGuire A, Capewell S, McMurray JJV. The current cost of heart failure to theNational Health Service in the UK. European Journal of Heart Failure. 2002;4:361–71. doi: 10.1016/s1388-9842(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Verhagen 1998 .Verhagen AP, de Vet HC, de Bie RA, Kessels GH, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. Journal of Clinical Epidemiology. 1998;51:1235–41. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- Vinson 1990 .Vinson JM, Rich MW, Sperry JC, et al. Early re-admission of elderly patients with congestive heart failure. Journal of the American Geriatrics Society. 1990;38:1290–5. doi: 10.1111/j.1532-5415.1990.tb03450.x. [DOI] [PubMed] [Google Scholar]


