Abstract
The vagus nerve is a major pathway by which information is communicated between the brain and peripheral organs. Sensory neurons of the vagus are located in the nodose ganglia. These vagal afferent neurons innervate the heart, the lung and the gastrointestinal tract, and convey information about peripheral signals to the brain important in the control of cardiovascular tone, respiratory tone, and satiation, respectively. Glutamate is thought to be the primary neurotransmitter involved in conveying all of this information to the brain. It remains unclear how a single neurotransmitter can regulate such an extensive list of physiological functions from a wide range of visceral sites. Many neurotransmitters have been identified in vagal afferent neurons and have been suggested to modulate the physiological functions of glutamate. Specifically, the anorectic peptide transmitters, cocaine and amphetamine regulated transcript (CART) and the orexigenic peptide transmitters, melanin concentrating hormone (MCH) are differentially regulated in vagal afferent neurons and have opposing effects on food intake. Using these two peptides as a model, this review will discuss the potential role of peptide transmitters in providing a more precise and refined modulatory control of the broad physiological functions of glutamate, especially in relation to the control of feeding.
Keywords: Vagus nerve, Nucleus of the solitary tract (NTS), Glutamate, Gut–brain signaling, Satiation, Food intake, CART, MCH Cardiovascular, Respiratory
1. Introduction
The vagus nerve provides a major bidirectional route of communication between the brain and peripheral organs. Afferent fibers of the vagus nerve convey sensory information from the gastrointestinal tract, heart, lung, liver, and pancreas to the central nervous system; while vagal efferent fibers are involved in motor function conveyed from the CNS to visceral organs. The pseudounipolar cell bodies of vagal afferents reside in bilateral nodose ganglia, and are involved in sensing multiple mechanical, chemical, osmotic, thermal, and possibly nociceptive stimuli. The total number of vagal afferent neurons has been found to be roughly similar across a number of different species and ranges from 16,000 to 18,000 perikarya, with the densest distribution of peripherally projecting fibers innervating the proximal GI tract. Tracing experiments demonstrate that the right and left nodose ganglia innervate slightly different peripheral sites [1,2]. Fibers of vagal afferent neurons also project to CNS neurons in the brain stem where they make synaptic connections with individual second order neurons in the nucleus of the solitary tract (NTS).
As a result of the large quantity of information transmitted by vagal afferent fibers it is unsurprising that they outnumber the vagal efferent fibers by approximately 9 to 1 [3]. Vagal efferent neurons carry parasym-pathetic motor-control fibers that are associated with the autonomic nervous system from the brainstem to visceral organs like the lungs, the heart, and the gastrointestinal tract. Vagal motor neurons are localized in two brain stem nuclei in the nucleus ambiguous (NA) and in the dorsal motor nucleus. They receive afferent inputs predominantly from NTS neurons, either as fiber projections from the NTS neurons or by sending their own dendrites into the NTS [4].
The vast array of information sensed by the vagus nerve from a large number of peripheral organs results in an extensive range of physiological functions, including a role in cardiovascular tone, respiratory tone, satiation, GI motility, digestion and absorption, emesis, and inflammation. The mechanism by which this information is communicated to the brain is not well defined. The release of the neurotransmitter glutamate from vagal afferent neurons has been demonstrated to play a role in a large number of these physiological functions, but the mechanisms by which this single neurotransmitter can regulate such an extensive list of physiological functions from a wide range of visceral sites remain unclear. The mechanisms of glutamate signaling will be addressed in this review. Extensive studies of the late 80s and 90s demonstrated that vagal afferent neurons express a range of peptides that can play a role in mediating some of the functions associated with vagal afferent neurons. Recently two novel putative peptide transmitters have been identified in vagal afferent neurons. These peptides are differentially expressed in vagal afferent neurons and are associated with opposing effects on feeding behavior. Using these two peptides as a model, this review will discuss the potential role of peptide transmitters in providing a more precise and refined modulatory control of the broad physiological functions of glutamate, especially in relation to the control of feeding.
2. Evidence for a role of glutamate in vagal afferent signaling in the NTS
The view that glutamate is the primary neurotransmitter of vagal afferent signaling to the NTS is widely shared [5–10]. This view is grounded in substantial anatomical, electrophysiological, and pharmacological evidence. Glutamate immunoreactivity can be observed in approximately 60% of neuronal cell bodies in the nodose ganglion [11–13], as well as axons in the tractus solitarius and terminals in the NTS [12]. Nodosectomy, which removes the vagal afferent cell bodies, results in a 40% reduction in the high-affinity uptake of glutamate in the medial NTS of rats [14]. Furthermore, immunohistochemical data suggests that both types of vesicular glutamate transporter, VGLUT1 and VGLUT2, required for packaging cytoplasmic glutamate into vesicles, are present in vagal fibers and terminals in the NTS since they are significantly reduced following nodose ganglionectomy [15]. Interestingly, vagal afferents fibers retrogradely labeled from the stomach are predominantly VGLUT2 positive, while fibers retrogradely labeled from the heart are VGLUT1 positive [16]. In summary, the machinery required for uptake and packaging of glutamate is present in vagal afferent neurons.
Indirect evidence for a signaling role of glutamate in the NTS was demonstrated by intracellular recordings in slices of rat brain. Postsynaptic potentials evoked in NTS neurons were identified as predominantly excitatory [17]. Furthermore, microinjection of [3H]-D-aspartate, a biochemical marker for excitatory amino acid-utilizing neurons, into the NTS of rats, retrogradely labels to cell bodies of nodose ganglia neurons [18]. Direct measurement of glutamate concentration in the NTS by microdialysis demonstrated that glutamate levels increased in the NTS following electrical [19,20], chemical [11,21], and mechanical stimulation of the vagal afferent fibers [22]. Unilateral nodosectomy reduced NTS levels of glutamate on the ipsilateral, but not the contralateral [23,24]. Together these data suggest that many of the vagal afferent fibers projecting to the NTS store and release glutamate, consistent with a transmitter role for glutamate. Although it should be noted that at least one group repeatedly failed to demonstrate changes in glutamate concentration in the NTS by microdialysis in response to vagal afferent electrical stimulation or nodosectomy [25–27].
Importantly, the receptors required for glutamate signaling can be found on postsynaptic NTS neurons. Over the years, molecular cloning of cDNA encoding glutamate receptors has revealed multiple groups of ionotropic glutamate receptor subunits [28]. These included six N-methyl-D-aspartate (NMDA) receptor subunits (NR1, NR2A to NR2D, and NR3), four α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) receptor subunits (GluR1–GluR4), and five kainate (KA) receptor subunits (GluR5 to GluR7, KA1, and KA2). Multiple G-protein-coupled receptors (GPCR), known as metabotropic glutamate receptors, have also been identified [29]. All of these receptors can be found in the NTS [30–32]. Both microinjection of glutamate into the NTS and vagal afferent stimulation can induce a wide range of physiological responses and these effects can be blocked using glutamate receptor antagonists [22,33–36]. Therefore there is substantial evidence supporting the concept that glutamate is a major neurotransmitter of vagal afferent neurons in the NTS that mediates physiological responses to peripheral sensory information (see Fig. 1).
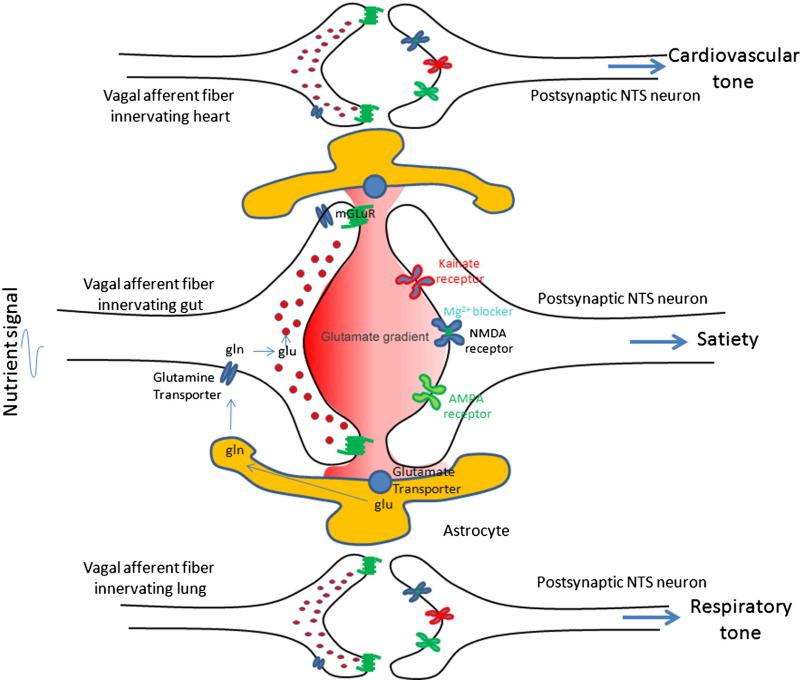
Fig. 1.
The role of glutamate in vagal afferent signaling. Vagal afferent fibers terminate on individual NTS neurons, enabling peripheral signals to mediate appropriate physiological responses (i.e. vagal afferent neurons innervating the heart controls cardiovascular tone; vagal afferent neurons innervating the gut controls satiation). Glutamate packaged in small vesicles in vagal afferent fibers is released into the synaptic cleft in the NTS in response to peripheral signals from visceral organs. The metabotropic glutamate receptor expressed on the presynaptic vagal afferent fibers regulates glutamate. Glutamate activates one or a combination of different ionotropic classes of receptors on postsynaptic NTS neurons mediating an appropriate physiological response. Astrocytes control temporal and spatial availability of glutamate, and minimize spillover, by regulating glutamate re-uptake. Glutamate transporters on astrocytes remove glutamate from the synaptic environment. Glutamate is converted to the inactive glutamine. Glutamine is released from astrocytes and transported into presynaptic neurons and repackaged into vesicles.
3. Evidence that glutamate is involved in feeding
Bednar et al. [37] were the first to provide evidence that glutamate release from vagal afferent fibers in the NTS could mediate feeding behavior. They found that in micropunches of the medial NTS, fasted rats had reduced glutamate concentrations; while intraperitoneal (IP) injections of the gastrointestinal hormone cholecystokinin (CCK) or ingestion of sucrose reinstated glutamate levels. Furthermore they reported that IP injection of the non-competitive NMDA receptor antagonist, MK801, facilitated sucrose ingestion and antagonized CCK-induced satiation. Ritter and Burns published a series of papers expanding on the idea that NMDA receptors mediate vagally-induced ingestion. They demonstrated that systemic NMDA receptor blockade increased intake of solid food or 15% sucrose solutions after an overnight fast, without affecting water intake in rats [38]. Intake of 0.2% saccharin solution, which has no nutritive benefit, was not increased with MK801 administration [39]. They reported that IP MK801 did not cause meal initiation, but rather increased the size and duration of meals that were already initiated [39]. Most convincingly they demonstrated that subdiaphragmatic vagotomy prevented IP MK-801-induced increases in meal duration [40], suggesting that glutamate activation of NMDA receptors mediates vagal transmission of satiety signals from the GI tract.
A number of findings that followed this work brought into questioned the site of action of systemic MK801. It became clear that IP MK801 did not inhibit satiation caused by vagal afferent glutamate activation of NTS neurons, but rather caused increased food intake by inhibiting activation of vagal efferent neurons leading to acceleration of gastric emptying. Two separate studies demonstrated that the stomach was important in mediating the effects of systemic MK801. Pretreatment of IP MK-801 failed to attenuate satiation of macronutrients in which the stomach was bypassed by infusion into the intestine [41] and also failed to prevent the satiating effects of exogenous CCK, known to be released from enteroendocrine I cells predominantly located in the duodenum [42]. It was then found that inducing vagal afferent lesions with capsaicin failed to prevent MK801 induced increases in meal duration; while inhibiting both vagal afferent and efferent signaling, by either subdiaphragmatic vagotomy [40,43] or lesioning the dorsal vagal complex [44], blocked the effects of systemic MK801. This suggested that vagal efferent neurons were the target site of IP MK801 (see Fig. 2). Finally, it was demonstrated that IP injection of MK801 acting on vagal efferent neurons accelerated gastric emptying [45,46].
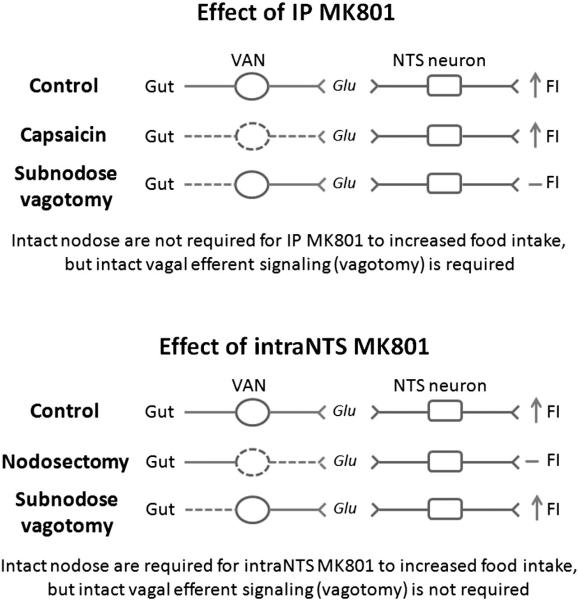
Fig. 2.
Systemic MK801 and intraNTS MK801 activate different pathways. Both intraperito-neal and intraNTS administration of MK801 increase food intake. An intact vagus nerve is required for systemic MK801 as demonstrated by the fact that vagotomy inhibits the increases in food intake caused by IP MK801. However, vagal afferent neurons, as demonstrated by capsaicin treatment, are not required for the orexigenic effects of systemic MK801. Therefore vagal efferent neurons modulate the feeding effects of IP MK801. Conversely, intraNTS MK801 was inhibited by nodosectomy, but not by sub-nodose vagotomy. This suggests that vagal afferent neurons modulate the feeding effects of intraNTS MK801. Thus, systemic MK801 and intraNTS MK801 administration activate different pathways.
Crucially endogenous glutamate was demonstrated to be released from vagal afferent neurons and inhibit food intake by activating postsynaptic NTS neurons. Firstly Covasa et al. demonstrated that 4th ventricle injection of MK801 increased food intake through a mechanism independent of gastric emptying [47]. Multiple studies subsequently reported that microinjection of NMDA receptor antagonists into the medial NTS of fasted rats increased meal size [48–53], providing evidence that at the site of vagal afferent fiber termination, endogenous glutamate was present and involved in satiation. Interestingly cannula tips located tenths of millimeters outside of this region failed to increase feeding [48]. MK801 has been reported to have off-target effects, interacting with other ion channels; however the NMDA receptor antagonists, AP5, and a NR2 subunit antagonist that distinguishes between different types of NMDA receptors, have also been shown to increase food intake in rats when injected into the medial NTS [49,50]. CCK-induced satiation, known to activate a vagal afferent pathway, was also found to be abolished by NTS injection of MK801 [51]. Neuro-anatomical studies demonstrated that exogenous CCK administration activated neurons in the medial NTS, and that activation of these neurons was abolished with NMDA receptor antagonists [51,53]. Importantly, unlike systemic administration of MK801, the orexigenic effects of intraNTS MK801 were not inhibited by subnodose vagotomy. Taken together with findings that unilateral nodosectomy prevented increased meal size of ipsilateral, but not contralateral, injection of MK801 into the medial NTS [52], this conclusively demonstrated that vagal afferent fibers release of endogenous glutamate activates NMDA receptors in the medial NTS to modulate satiation (see Fig. 2).
The role of non-NMDA receptor of the hindbrain in the control of food intake is less clear. Anatomical studies indicate that most if not all NTS neurons express non-NMDA receptors [54]. Although, gastric distension or duodenal nutrient stimuli activate NTS neurons that express the AMPA receptors, GLUR2/3 [55], pharmacological studies have failed to demonstrate a role for non-NMDA receptors in satiation. Inhibiting both AMPA receptor and kainite receptors in the hindbrain using the antagonist, 1,2,3,4-tetrahydro-6-nitro-2,3-dioxobenzo[f] quinoxaline-7-sulfonamide (NBQX), was found to potently suppress sucrose, chow, and water intake in a dose-dependent manner in both fed or fasted rats. In this thorough study the authors demonstrate that NBQX injection into the NTS does not cause conditioned taste aversion or motor deficiency [55]. Instead the authors suggest that reduced sense of taste, caused by NBQX inhibiting taste-induced activity of rostral NTS neurons, could be responsible for the reduction in licking frequency. Although from these studies it cannot be ruled out that endogenous glutamate activation of AMPA receptor results in increased food intake. Irrespective of the mechanisms by which intraNTS NBQX inhibits food intake, the data indicates that despite virtually all NTS neurons expressing non-NMDA receptors, these are not involved in mediating the satiating effects of vagal afferents from the gastrointestinal tract. Instead endogenous glutamate in the NTS requires NMDA receptors to mediate satiation.
4. Other physiological functions mediated by glutamate
As alluded to above, vagal afferent fibers convey an impressive array of information to the NTS. Vagal afferent fibers innervate the oral cavity, esophagus, stomach, small intestine, liver, pancreas, heart, and lungs and are therefore involved in a number of different physiological functions. There is a large body of evidence suggesting that glutamate is the primary neurotransmitter released from vagal afferent terminals to modulate respiration (see [56] for review), cardiovascular reflexes (see [57] and [58] for review), GI motility (see [6] for review), ingestion (see [4] for review) as well as provide information to the brain about the types (lipid, carbohydrate, and protein), quantity, and location of these nutrients within the gastrointestinal tract resulting in satiation, and may also be involved in taste perception [59] (see Table 1). This is a very broad and extensive range of information to be conveyed to the brain by one neurotransmitter. How can all of this information be differentially relayed to the brain by glutamate?
Table 1.
| Amino acid based transmitters | Expression in nodose ganglia (NG) and vagal afferent fibers | Peripheral signaling | Functional role in the NTS | Intact vagal afferents required for function in NTS |
|---|---|---|---|---|
| Glutamate | •Glutamate immunoreactivity has been observed in NG and vagal afferent fibers [11-13] •Vesicular glutamate transporters (VGLUT) are expressed in vagal afferent fibers [15-16]. •Nodosectomy reduces glutamate uptake by 40% [14]. |
•VGLUT1 and VGLUT2 expressing VAN were retrogradely labeled to the trachea [173]. •VGLUT1 vagal afferent fibers retrogradely label the heart [16]. •VGLUT2 vagal afferent fibers retrogradely label the gut [16]. |
•Cardiovascular reflex [57], •Respiration [56] •Satiation (see this review), •Ingestion [4], •Motility [6], •Taste [59], •Inflammation [175] •Emesis [176] |
Nodosectomy prevents endogenous glutamate induced satiation [52] |
| Gamma-aminobutyric acid (GABA) | GABA immunoreactivity has been observed in NG [23] and vagal afferent fibers in the NTS [155] | Unknown | •GABA injection into NTS increases blood pressure [156] •GABAA receptor antagonist increases aortic depressor nerve stimulation [158,159] •GABAB receptor antagonist increases blood pressure [160] and attenuates reduction in blood pressure following electrical activation fo the aortic depressor nerve [161] |
Unknown |
| Nitric oxide (NO) | Nitric oxide synthase (NOS) expressed in nodose ganglia neurons [140,141]. and vagal afferent fibers in the NTS [142-145] | •3% of neuronal NOS (nNOS) expressing VAN were retrogradely labeled to the trachea [174], •A large proportion of nodose ganglia neurons retrogradely labeled from the aorta depressor nerve co- localize with nNOS [146]. •nNOS was expressed in about a third of all vagal afferent neurons projecting to the stomach [177] •eNOS is ubiquitously expressed in VAN [142] |
•NO donor into the NTS decreases [147,148] while NOS inhibitor increases [149] arterial pressure and heart rate. •nNOS inhibitor (L-NAME), but noteNOS inhibitor (L-NIO) administered to gastroesophageal vagal afferent fibers enhanced mechanosensitivity [177]. The role of NO on glutamate release in the NTS was not tested therefore it remains unclear ifthis is a direct effect of NO or an inderect effect of modulating glutamate release. |
Unknown |
| Acetylcholine | The enzyme choline acetyltransferase required for acetylcholine production is expressed in nodose ganglia neurons [178,179] | Unknown | Microinjection of nicotinic acetylcholine receptor agonist, nicotine, into the NTS produced a dose- dependent decrease in BP and HR[180] | Unknown |
| Dopamine | Immunoreactivity of both dopa decarboxylase [181 ] and tyrosine hydroxylase, required for dopamine synthesis, has been observed in NG [182,199] | Tyrosine-hydroxylase containing vagal afferent neurons project to the esophagus and stomach [183] | Microinjection ofdopamine into the NTS increased blood pressure and heart rate [184] | Unknown |
| Serotonin | Serotonin (5-HT)-like immunoreactivity has been detected in cell bodies ofthe nodose ganglia [185] and vagal afferent fibers [186,187,197] | Unknown | •Blood pressure and heart rate are decreased by low doses of 5HT but increased by high doses of 5HT microinjected into the NTS [188]. •5HT containing vagal afferent fibers in the NTS come into closecontact with NTS neurons implicated in respiration [189] |
Unknown |
| Peptide based transmitters | Expression in Nodose ganglia (NG) and vagal afferent fibers | Peripheral signaling | Functional role in the NTS | Intact vagal afferents required for function in NTS |
| Cocaine- and amphetamine-regulated transcript (CART) | Protein and mRNA for CART was identified in NG and in vagal afferent fibers in the NTS [82-84]. | •CART is expressed in 17% of neurons that project to the stomach and 41% of vagal afferent neurons that project to the small intestine [83] •Protein expression was demonstrated to be differentially regulated in response to nutritional status: expressed postprandially in NG and vagal afferent fibers in the NTS [82] |
•Inhibiting endogenous CARTin the NTS stimulates food intake [98]. •CARTacts as an autocrine regulator of gene expression in vagal afferentneurons [86]. |
Nodosectomy prevents endogenous CARTinduced satiation [98] |
| Melanin concentrating hormone (MCH) | Protein and mRNA for MCH was identified in NG and in vagal afferent fibers in the NTS [102,9,111-113]. | Protein expression was demonstrated to be differentially regulated in response to nutritional status: expressed under fasting conditions in NG [82,102] | •Blocking endogenous MCH in the NTS inhibits food intake [98]. •MCH1 receptors are expressed by vagal afferent neurons in response to prolonged fasting, suggesting MCH may act as an autocrine regulator of vagal afferent signaling [7,99]. |
Nodosectomy prevents endogenous MCH induced food intake [98] |
| Substance P (SP) | •mRNA for preprotachykinin A (precursor to SP, NKA) found in nodose ganglia by in situ hybridization [190] and SP immunoreactivity was also observed in nodose ganglia [162,164,199] •Unilateral nodosectomy reduces SP immunoreactive vagal afferent fibers in the NTS [165]. |
•SP did not colocalize with nodose ganglia retrogradely labeled from the lung under basal conditions [191], but acute allergen exposure increases SP mRNA in vagal afferen neurons [166]. •Vagal afferent neurons retro-gradely labeled to the carotid sinus and aortic nerve colocalize extensively with SP [124] and activation of the aortic nerve increases SP expression in the NTS [125,126] |
•Injection of SP into the NTS induced •central apnea [172] •mixed effects on blood pressure and heart rate, low doses decrease both[132,133,135,136,171], or having no effect on both [128,132] •Microinjection of SP antagonist into the NTS increases blood pressure and heart rate [133], •Ablation of NTS neurons that express NK1 receptor prevents the gain of the baroreflex [134]. |
Substance P dose dependently decreased the frequency but not amplitude of excitatory postsynaptic currents in NTS neurons receiving monosynaptic vagal afferent input from lungs and airways [212]. This suggests that SP released from vagal afferents into the NTS can modulate respiratory tone by inhibiting pre- synaptic release of glutamate. |
| Neurokinin A (NKA) | •mRNA for preprotachykinin A (precursor to SP, NKA) found in nodose ganglia by in situ hybridization [190] •Immunoreactive NKA was observed in nodose ganglia neurons [163] •Unilateral nodosectomy reduces NKA immunoreactive vagal afferent fibers in the NTS [165]. |
• Increased levels of preprotachykinin-A mRNA which encodes NKA were found in nodose ganglia following 24 h allergen challenge in guinea pigs [166]. | •Microinjection of NKA into the NTS dose- dependently causes prompt, transient hypotension and brady- cardia [165]. •NKA microinjection into the NTS reduced respiratory frequency and increased tidal volume [167,168]. |
Unknown |
| Calcitonin gene-related peptide (CGRP) | CGRP immunoreactivity in NG and vagus nerve colocalizes with Sub stance P[192,163] and in vagal afferent fibers [193] | •CGRP expressed in NG retrograde-ly labeled to lung [191,194] •In the nodose ganlia the majority of the retrograde labeled nerver cell bodies from the esophagus stored CGRP [192] |
Lesion of the area postrema/nucleus of the solitary tract (AP/NTS) attenuates CGRP-induced satiety [193] | Unknown |
| Cholecystokinin (CCK) | •CCK immunoreactivity was demonstrated in NG [118,160,195,196,198,199] •No preproCCK mRNA was identified in nodose ganglia under basal conditions [187] •There are reports that the antibody used mayalso bind CGRP [160]. |
•CCK was absent under basal condition but increased in response to nerve damage [200] •No change in response to food deprivation or high fat feeding [200]. |
Unknown | Unknown |
| Galanin | Galanin immunoreactivity and mRNA have been reported in nodose ganglia [201,202] | Retrogradely labeled galanin-positive vagal afferent neurons project to the stomach [203] | •Injection of galanin into the NTS: •stimulate feeding [204] •reduces blood pressure [205] •increases heart rate [205] •Galanin inhibits mechanosensitivity of vagal afferents, possibly by acting as an autocrine regulator [203]. |
Unknown |
| Neuropeptide Y (NPY) | In situ hybridization [190] and immunoreactivity [205] confirmed the presence of NPY in nodose ganglia | Unknown | Possible role in modulating blood pressure [206] | Unknown |
| Somatostatin | In situ hybridization [190] and immunoreactivity [163,199] confirmed the presence of somatostatin in nodose ganglia | Unknown | Inhibition of somatostatin receptor 5 in the NTS stimulates gastric emptying [207] | Unknown |
| Vasoactive inhibitory peptide (VIP) | Caudal distribution of VIP immuno- reactivity in vagal afferent neurons [163,199] | Vagal damage (ie. vagotomy) and inhibiting vagal transport significantly increased VIP mRNA and immunoreactivity [208,209,210] |
VIP injected in the Nucleus Tractus Solitarius- Dorsal Motor Nucleus complex reduced jejunal alanine and water absorption, and increased gastric acid release [211] | Unknown |
| Brain-derived neurotrophic factor (BDNF) | Abundantly expressed in adult vagal afferent neurons [213] | In cultured neurons. BDNF release is regulated by physiological patterns of baroreceptor activity [213] | Possible role in mediating baroafferent synapses [214] | Unknown |
This question has not been directly addressed in the literature, mostly because it is not currently possible to determine the functional role of individual neurons in the NTS. Despite the lack of strong empirical evidence, the prevailing view is that fibers projecting from specific visceral sites activate precise neurons in the NTS with individual function. For example, vagal afferent fibers innervating the gut will sense distension of the stomach, send an action potential along the axon which releases glutamate onto a postsynaptic NTS neuron involved in motility, which will control gastric emptying by activating the vago-vagal reflex. Therefore according to this hypothesis it is important that the correct neuron is activated to prevent inappropriate physiological response requiring both tight spatial and temporal control of glutamate release. How does this occur?
As the prototypical fast neurotransmitter, glutamate is transiently released in short bursts from afferent fibers following depolarization caused by peripheral stimuli [60]. A negative feedback system, by which glutamate activates metabotropic glutamate receptors on the presynaptic cleft, prevents prolonged release of glutamate [61]. In addition, glutamate is rapidly removed from the synapse by glutamate transporters predominantly found on glial cells that surround the synapse [62]. This process is highly efficient since it happens against the electrochemical gradient and allows glutamate to be recycled [62]. Most importantly the density of these transporters can control the concentration of glutamate in the synapse, the length of time glutamate is available to bind to ionotropic glutamate receptors on the post-synaptic cleft, and reduce glutamate diffusion out of the synapse, thereby limiting activation of neighboring neurons. Thus, at least in theory, tight spatial and temporal control of glutamate release from fibers projecting from specific visceral sites can result in precise activation of individual neurons in the NTS to mediate a specific physiological response.
There is some evidence for viscerotopic distribution in the NTS [63–66]. This is especially true of the gastrointestinal tract, with rostrocaudal organization in nodose ganglia neurons and the NTS mapping the length of the alimentary tract [64]. As food travels through the alimentary tract, more caudal regions of the NTS are activated [66]. Certainly this may provide some insight as to how subdiaphragmatic vagal afferents can provide information about the location of the nutrients within the gastrointestinal tract to the brain. However, there is no clear pattern of organization for vagal afferent fibers projecting from different visceral afferent regions; afferents innervating the lung, heart and gut are all bundled together and terminate in similar sub-regions of the NTS [65]. Furthermore, there does not seem to be viscerotopic distribution of postsynaptic NTS neurons with different functions. Neurons receiving different sensory inputs from the subdiaphragmatic vagus nerve, baroreceptors or chemoreceptors all localize within the same sub-region of the NTS [67]. For this reason the prevailing view is that fibers projecting from specific visceral sites activate precise neurons in the NTS [68]. This would explain how the same neurotransmitter can produce different physiological responses to peripheral signals in the NTS and provide a wide range of information about the nutritional content of food and its transit through the gastrointestinal tract. Further work is still required to determine the mechanisms by which fibers from nodose ganglia neurons project to individual neurons in the NTS during development in light of the seeming lack of organization of these second order neurons. Methodical mapping of these neurons within the NTS may provide some insight.
5. Rationale for a role of other neurotransmitters
There are three flaws that exist with the concept of glutamate as the predominant neurotransmitter of vagal afferent neurons:
NMDA receptors require a depolarizing event in addition to glutamate in order to be activated, and therefore glutamate alone is insufficient to regulate satiation.
Since NTS neurons with different functions are intermingled in the same regions, any spillover of glutamate out of the synapse would cause inappropriate functional responses to peripheral signals
Meal size and duration vary greatly across the day even when the same food with the same nutrient content is ingested, which suggests that a greater plasticity of signaling is required than can be provided by glutamate alone.
In this section each of these points will be addressed in more detail and the rationale for the involvement of modulatory peptides released from vagal afferent alongside glutamate will be discussed as a requirement for appropriate glutamate signaling.
Glutamate binding to NMDA alone is insufficient to activate neurons. At resting membrane potential, NMDA receptors are blocked by magnesium ions; an additional membrane depolarization event is required for the Mg2+ block to be relieved and allow those NMDA receptors which have glutamate bound to open [69]. The classical view is that glutamate acting on non-NMDA receptors would depolarize the neuron and enable glutamate activation of NMDA receptors. However, as discussed above, non-NMDA receptor antagonists are not involved in satiation. Therefore glutamate alone is insufficient to result in satiation and something else is required to depolarize the postsynaptic NTS neurons. Modulatory neuropep-tides co-released with glutamate from vagal afferent fibers provide an elegant solution to this problem. These peptides could be released alongside glutamate in response to a specific peripheral stimulus so that they can 1) modulate glutamate-induced NMDA activation by modifying the membrane potential, and 2) target the intended postsynaptic neuron, without activating neighboring quiescent neurons that lack the receptor for the neuropeptide. The presence of inhibitory and stimulatory modulatory peptides could have opposing effects on the membrane potential of postsynaptic neurons and either prevent or enable NMDA activation in the presence of glutamate. It is also possible that modulatory peptides regulate non-NMDA receptors, which would reconcile the anatomical evidence that most NTS neurons express these receptors, with the pharmacological data suggesting that they are not involved in satiation.
Despite the very high numbers of glutamate transporters surrounding synapses, evidence has emerged that glutamate can leak out from its synapse and diffuse to activate receptors on inactive neighboring synapses [70]. This process, known as spillover, may be problematic in the NTS where multiple neurons with different function intermingle. Spillover has predominantly been identified in hippocampal slices, however glutamate spillover in the NTS has been hypothesized in the past [54,71]. Modulatory peptides released from vagal afferent neurons alongside glutamate could prevent unwanted side-effects of spillover onto neighboring neurons, by depolarizing only the subset of NTS neurons that encode the appropriate neuropeptide transmitter.
Finally meal patterns in ad libitum fed animals are highly variable even in response to the same nutrient composition. Ingestion of the same chow diet (or any predefined nutrient composition) should result in the same release of glutamate in response to nutrient activation of taste receptors, swallowing, gastric distension, release of gastrointestinal hormones and absorption, resulting in the same amount of satiation. So every meal should terminate at the same time. However, this is not the case, instead each meal is heterogeneous. These observations suggest a certain amount of plasticity to gut–brain signaling must occur and this cannot be attained from glutamate alone. Instead plasticity in the expression and release of modulatory peptides could address this problem.
The advantage of neuromodulators participating in glutamate activation in the NTS is that they can be released alongside glutamate from vagal afferent fibers in response to the same visceral stimulus, and provide close regulation of glutamate-induced effects. Modulatory peptide transmitters could regulate glutamate activation of NTS neurons in multiple ways. Presynaptically, modulatory neurotransmitters could control the duration of glutamate secretion from vagal afferent neurons. Since neuropeptide transmitters remain in the synapse longer than glutamate, this could even allow them to affect the release of glutamate in response to future peripheral stimuli. Post-synaptically, they could alter the membrane potential of postsynaptic NTS neurons to control glutamate-induced activation of NMDA receptors. These modulatory peptides could alter glutamate transporter expression on glial cells in the NTS to reduce/increase glutamate re-uptake to change the duration and site of action of glutamate. Finally, they could bind neighboring quiescent neurons to regulate the convergence of different physiological processes. This would enable a primary site of crosstalk across multiple organ systems. For example, Gilbey et al. have demonstrated that there is a significant overlap in respiration and cardiovascular signaling to maintain an appropriate balance of oxygen and carbon dioxide in response to changes in physiological demand [72]. It could be possible for peptides to induce all or some of these modulatory effects at the same time, or for one peptide to modulate one aspect of glutamate signaling while another peptide to modulate a different aspect.
Neuropeptides are well suited to a modulatory role because they have slow, prolonged effects compared to classical neurotransmitters. While glutamate can be rapidly removed from the synapse, no evidence of peptide re-uptake has been observed [73]. Instead, neuropeptides are slowly removed from the extracellular space by diffusion and breakdown by proteases [73]; some neuropeptides have been shown to have half-lives of 20 min in the brain [74]. Neuropeptide transmitters also activate GPCR, which produce slower signals than fast synaptic transmission. Unlike glutamate, which is recycled at the synapse, neuropeptides are synthesized in the nodose ganglia and are transported by fast axonal transport [73]. There is evidence that peptide levels may vary considerably in response to different stimuli [73], this makes them ideal modulators of fast neurotransmission. Unsurprisingly, neuropep-tides are found in different vesicles to classical neurotransmitters. Neuropeptides are found in sparse large dense core vesicles, while classical neurotransmitters are contained within highly abundant small synaptic vesicles that occupy a large portion of the axon terminal [75,76]. It is unclear whether these vesicles are released together or separately; although, there is evidence that these can be differentially released [77]. It was reported that neuropeptides in large dense core vesicles were predominantly released outside of the synapse [78,79]. However evidence exists to support the idea that neuropeptides are also released at synapses [80]. More recently, using novel fluorescent probes, it has been found that although dense core vesicles were not enriched in synaptic terminals, they were preferentially released at the synapse [81]. In this study, it was further demonstrated that the Munc13 family of proteins, essential for the assembly and release of classical neurotransmitters from synaptic vesicles, also facilitate secretion of neuropeptides at synaptic terminals [81].
Although neuropeptides are preferentially released at the synapse, they are clearly also released extrasynaptically. We can only speculate the reason for extrasynaptic release of neuropeptides. Perhaps this enables neuropeptides to increase the area over which they can exert their effects [78]. Extrasynaptic release would blanket a larger area in the NTS than glutamate while only activating those NTS neurons that were coded with the appropriate peptide receptors. This would be beneficial as it could modulate glutamate release from neighboring presynaptic vagal afferents, enable modulation of postsynaptic gluta-mate activation prior to the complete transport of neuropeptide from the cell body to the synapse, and/or enable coordinated activation of multiple postsynaptic neurons coded with the peptide receptor. Understanding the signals involved in promoting synaptic or extrasynaptic release of neuropeptide may provide insight into the functional importance of these different mechanisms.
6. Evidence for a role of neuropeptides in satiation
The idea of neuromodulators is supported by the fact that multiple peptides are expressed in nodose ganglia neurons (see Table 1). Immunoreactivity in NTS fibers for all of these peptides is significantly reduced by vagal ablation or nodosectomy and receptors for these peptides have been found in the NTS. Importantly, microinjection of each putative neuropeptide transmitter into the NTS produces a distinct set of physiological responses, while injection of antagonists to their receptors inhibits these physiological effects. Interestingly, the majority of these neuropeptides have had conflicting reports about their function when microinjected into the NTS, which supports the idea that they are involved in modulating rather than mediating visceral function. In the next section, evidence for the role of two novel neuropeptide transmitters expressed in vagal afferent neurons will be discussed as putative modulators of food intake.
6.1. Cocaine and amphetamine regulated transcript
Cocaine and amphetamine regulated transcript (CART) has been observed in approximately half of the neurons in the middle and caudal regions of the nodose ganglion [82–84]. Pinching experiments provide evidence that CART is transported along the vagus nerve [83]. Consistent with this idea, dense populations of CART fibers have been observed in the commissural and medial parts of the NTS [84,85], and these fibers were greatly reduced in response to supranodose vagotomy [84].
There is evidence that CART in vagal afferents plays a role in mediating the satiety induced by gastrointestinal signals. Retrograde labeling experiments demonstrate that CART positive neurons in the nodose ganglia innervate predominantly the duodenum, but also to a lesser extent the stomach [84]. Importantly CART extensively co-localizes with CCK1 receptors on vagal afferent neurons [83] and IP administration of exogenous CCK increases the number of CART positive cells in the nodose ganglia [82]. Postprandial release of CCK increases the number of neurons expressing CART and this is inhibited following administration of a CCK1 receptor antagonist [82]. Administration of CCK to cultured primary vagal afferent neurons dose-dependently induced CART synthesis and release [86]. In support of the idea that CART could mediate the satiating effects of the gastrointestinal hormone CCK, IP administration of CART was found to prolong the satiating effects of exogenous CCK when administered together in rats after a short fast [86].
In addition to CCK, other gastrointestinal hormones have been implicated in regulating CART expression in nodose ganglia neurons. In cultured vagal afferent neurons leptin was found to potentiate CCK-induced neuronal expression of CART [87]. Leptin is released from chief cells of the stomach in response to nutrients, as well as from adipocytes in proportion to total body fat, however it remains unclear which site of leptin release is most important for the regulation of CART expression in vagal afferent neurons. The orexigenic gastrointestinal hormone ghrelin, released from A/X enteroendocrine cells of the stomach in the absence of the nutrients, was found to inhibit both the potentiating effects of leptin as well as the effects of CCK on CART expression in cultured vagal afferent neurons [82]. Orexin A, an orexigenic peptide expressed by a population of hypothalamic neurons with terminals that project to the NTS, has also been demonstrated to inhibit CART synthesis in response to CCK [71]. Thus, CART synthesis and release from vagal afferent neurons has been demonstrated to be regulated by hormones associated with feeding behavior.
There is evidence that both endogenous and exogenous CART inhibits food intake in the CNS. CART has been localized to brain regions associated with feeding behavior [85] and there is a large body of literature demonstrating intracerebroventricular (ICV) injection of CART dose-dependently reduces food intake [88–92]. Vrang et al. demonstrated that among the many different regions of the brain activated by ICV administration of CART, there was significant and prominent activation of NTS neurons [89]. Fourth ventricular injection of CART, to activate hindbrain neurons, reduces gastric emptying and inhibits gastric acid secretion [90,93], while stimulating colonic motility [94]. Hindbrain stimulation with exogenous CART also reduces the short-term intake of a liquid meal [95,96] independently of its effects on gastric emptying [96]. Interestingly, plugging the cerebral aqueduct between the third and fourth ventricles blocks the anorectic effect of ICV injection of CART [97], leading to the hypothesis that the anorectic properties of CART are solely mediated by the hindbrain. Microinjections of CART directly into the medial NTS was not found to reduce food intake in fasted rats [84]; however inhibiting endogenous CART, using an antibody against CART, has been demonstrated to prevent satiation in rats fed ad libitum [98] and preliminary data suggests that the endogenous CART requires fully functional vagal afferent neurons [99]. Crucially, silencing of CART in nodose ganglia neurons in vivo was found to abolish CCK-induced satiation [100], suggesting CART plays an important role in mediating the satiating effects of CCK.
To summarize, CART is expressed and released by vagal afferent neurons in response to a meal. CART is transported along vagal fibers, silencing CART expression in vagal afferent neurons prevents CCK-induced satiation, and endogenous CART in the NTS inhibits food intake. Taken together these data strongly suggest that CART is a novel neuro-peptide transmitter expressed by vagal afferent neurons that is involved in satiation. The mechanisms by which CART mediates its effects remain unclear, partially due to the fact that the CART receptor has not yet been identified. We know that CART can act presynaptically on vagal afferent fibers in the NTS [86] and there is some limited data suggesting that CART and glutamate can interact, at least in a spinal cord slice preparation. In this preparation, CART was found to increase depolarization induced by NMDA-, but not AMPA, in substantia gelatinosa neurons [101]. Therefore it is possible to speculate that a similar mechanism may be involved in the NTS, whereby CART could depolarize postsynaptic NTS neurons to enable glutamate-induced NMDA receptor activation leading to satiation.
6.2. Melanin concentrating hormone
Melanin concentrating hormone (MCH) has recently been identified in mid- and caudal parts of the nodose ganglia [102]. MCH expression in vagal afferent neurons has been demonstrated to be regulated by the feeding state in rats; MCH abundance is high in the nodose ganglia of fasted rats, and is significantly reduced in response to a meal [102]. The vast majority of MCH-positive neurons in nodose ganglia co-localize with CCK1 receptor [102]. CCK was demonstrated to inhibit MCH expression in vagal afferent neurons; under fasting conditions, when MCH expression is high, CCK administration IP reduced the number of MCH-positive cells in the nodose ganglia. Conversely, postprandial reduction in MCH expression was reversed following administration of a CCK1 receptor antagonist [102]. In cultured vagal afferent neurons, CCK-induced downregulation of MCH was found to be prevented in cells transfected with a dominant negative mutant of the transcription factor cyclic-AMP response element binding protein (CREB) [82]. Exogenous administration of the gastrointestinal hormone ghrelin, released from the stomach in the absence of nutrients, attenuates postprandial-induced inhibition of MCH in nodose ganglia neurons [103]. Ghrelin was found to inhibit CCK-induced translocation of phosphoCREB to the nucleus, and therefore prevents active CREB from inhibiting MCH synthesis [82]. As a whole, these data suggest that MCH synthesis in vagal afferent neurons is upregulated by orexigenic gastrointestinal hormones and downregulated by the postprandial release of anorectic gastrointestinal hormones.
There is substantial evidence supporting the idea that MCH is involved in stimulating food intake [104–108]. Specifically, ICV injection of MCH increases food consumption [104,105] and ICV administration of MCH1 receptor antagonist reduces food intake [105]. MCH binds to a single G-protein-coupled MCH1 receptor that is widely expressed throughout the brain [109,110], including in the NTS. In the NTS of rats [7,111–113] and humans [114], MCH-positive fibers have been reported. Some MCH immunoreactive fibers are projections from hypothalamic MCH neurons; although the vast majority of the fibers are derived from another source [7]. The fact that NTS neurons activated by nutrient infusion into the stomach are targeted by MCH axons [7], suggests the possibility that a proportion of MCH fibers in the NTS are vagal afferents. This hypothesis is supported by unpublished data from our lab in which we found that nodosectomy significantly reduced MCH-positive fibers in the NTS.
In fasted animals, when endogenous MCH is highly expressed in vagal afferent neurons, fourth ventricular administration of MCH had no effect on food intake; however, it did reduce core body temperature without changing locomotor activity, suggesting a role in energy expenditure [7]. SNAP 94847, a MCH1 receptor antagonist, injected into the medial NTS inhibited food intake in fasted rats in a dose-dependent manner when compared to saline [98]. These data suggest that endogenous MCH is released from vagal afferent neurons into the NTS under fasting conditions, resulting in a net increase in food intake. MCH administration to brain slices decreased the frequency, but not the amplitude, of spontaneous excitatory post-synaptic currents [7], suggesting that MCH may inhibit glutamate release by acting presynaptically on vagal afferent fibers. Interestingly, MCH and MCH1 receptor highly co-localize in the same vagal afferent neurons [102], and the same gastrointestinal signals control their gene expression [102,103]. These presynaptic events are likely to be more pronounced in response to prolonged food withdrawal since MCH1 receptor requires greater than 24 h fasting before becoming fully observed in vagal afferent neurons [115]. MCH1 receptors have also been identified on postsynaptic membranes [110] and could indicate a role for MCH in inhibiting glutamate activation of NMDA receptors by preventing depolarization of the postsynaptic neurons. Evidence in DMV brain slices that MCH decreases the amplitude of excitatory postsynaptic current induced by stimulation of vagal fibers [7] supports this concept.
7. CART and MCH as modulators
Importantly, the same neuron can express both CART and MCH [82]. CCK acts as a gatekeeper that depresses synthesis of the orexigenic peptide MCH while rapidly stimulating expression of the satiety peptide CART (see Fig. 3). The ability of CCK to switch the neurochemical phenotype of vagal afferent neurons is potentiated by leptin and inhibited by ghrelin, and therefore these neurons express proteins that reflect the nutritional status of the animal. The differential expression of these peptide transmitters with opposing function on feeding behavior provides evidence for a previously unsuspected plasticity to gut–brain signaling in response to nutrient availability.
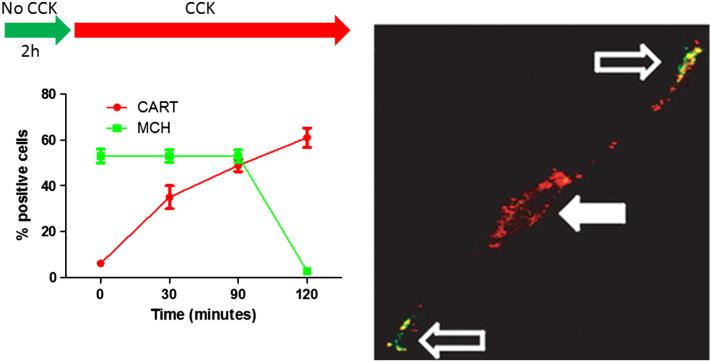
Fig. 3.
The CART/MCH switch in vagal afferent neurons. Primary vagal afferent neurons cultured in the absence of CCK predominantly express MCH. Administration of CCK rapidly increases CART expression. Approximately 90 min after CCK administration, both CART and MCH can be observed in different pools of vesicles within the same neuron. CART is predominantly expressed in the perinuclear space in the cell body with some vesicles appearing in the processes, while MCH is exclusively observed in the terminals about to be released. Within 2 h of CCK administration, cultured vagal afferent neurons express predominantly CART. Thus the same vagal afferent neuron can switch between the expressions of different neuropeptide transmitters based on signals from the gut. Modified from [76].
It is important to note that CART and MCH synthesis in response to nutritional status is unlikely to have an immediate effect on the current meal. Although vagal afferent neurons can rapidly switch their neuro-chemical phenotype (see Fig. 3), the transport of these neuropeptides from the cell bodies to the afferent terminals in the NTS will be a rate-limiting step in translating protein expression to function. We have evidence that anterograde labeled vagal afferent fibers in the NTS of fasted rats are devoid of CART, but CART immunopositive fibers appear within 2 h of refeeding [99]. This suggests that CART can be synthesized and transported from cell bodies to terminals in the NTS within 2 h. Interestingly, biotinylated dextran injection into the nodose ganglia, to anterogradely label the fibers in the NTS, requires 5–10 days to reach the terminals. Therefore there is a discrepancy in the rate of transport between CART and the anterograde label.
Although not specifically studied in vagal afferent fibers, pulse-chase radiolabeling experiments in sensory neurons of the eye and spinal cord have identified five classes of axonal transport that can be broadly categorized into fast and slow transport [116]. Fast axonal transport, which includes anterograde transport of neuropeptides in vesicles along microtubules, has been demonstrated to occur at a rate of 200–400 mm per day (1–5 μm/s) in mammals. Slow axonal transport carries cytoplasmic and cytoskeletal elements at a rate of 0.2–1 mm/day. Proteins destined for fast axonal transport are required to be sorted and packaged in the Golgi [117], which may explain the difference in the rate of transport between CART and anterograde labels (such as biotinylated dextran). Importantly, assuming neuropeptides are carried by fast axonal transport, this corroborates our finding that they can reach the NTS within 1–2 h. However this lag between peripheral stimulation and the availability of the neuropeptides at terminals in the NTS suggests that neuropeptides are most likely involved in mediating feeding behavior of subsequent meals (see Fig. 4). Energy restriction will result in vagal afferent neurons switching their neurochemical phenotype from CART expression to MCH expression. The longer the fast, the more MCH vesicles accumulate within vagal afferent fibers of the NTS. The first meal following a fast will result in the release of MCH alongside glutamate. MCH inhibition of glutamate will result in a reduction in satiation. Although further work is required to demonstrate the validity of the proposed hypothesis, it provides a rational for the observation that meal size and duration are increased following a fast. In response to the first meal, CART will be synthesized. As pools of MCH are depleted in the NTS, they will be replaced by CART, which will enable/potentiate the satiating effects of glutamate. Subtle changes in CART and MCH expression and release throughout the day provide some explanation for the variability in meal patters of chow fed rats (see Fig. 4).
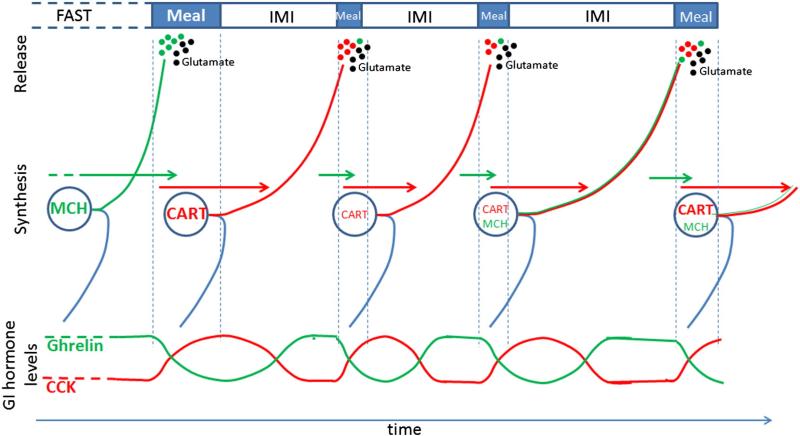
Fig. 4.
The role of CART and MCH in physiological feeding behavior. CART and MCH provide plasticity to the system enabling meal size and duration to change throughout the day. Importantly, CART and MCH synthesis and release in response to gastrointestinal signals will regulate feeding behavior of subsequent meals. MCH is synthesized in vagal afferent neurons and transported to the terminals during fasting periods; it is released into the NTS in response to a meal resulting in prolonged meal size and duration. CART is synthesized postprandially and transported during the intermeal interval; it is released during subsequent meals resulting in reduced meal size and duration. The switch in neurochemical phenotype of vagal afferent neurons enables tight control of glutamate-induced satiation. It remains unclear whether MCH is synthesized during intermeal intervals (IMI); however the release of both CART and MCH may explain the heterogeneity of meal size and duration over the course of the day in animals fed a diet with a set and unchanging nutrient composition.
8. The roles of transmitters in vagal afferent neurons
As discussed above, there is mounting evidence suggesting that CART and MCH play a role in the regulation of food intake, possibly by modulating glutamate-induced satiation. However visceral sensory neurons of the nodose ganglia express many different transmitters (see Table 1). These are expressed in small subpopulations of vagal afferent neurons that innervate different peripheral sites. Microinjection of the different transmitters expressed by vagal afferent neurons into the NTS results in a variety of physiological roles. Therefore, the plethora of peptides expressed by the different subpopulations of vagal afferent neurons may modulate the physiological effects of glutamate in response to peripheral stimuli. The idea that primary sensory neurons can exhibit pathway-specific neuropeptide expression has been previously reported. Gibbins et al. demonstrated that individual dorsal root ganglia neurons contain multiple neuropeptides and that different combinations of neuropeptides were expressed in neurons with different peripheral projection [118]. Interestingly McMahon et al. found that changing the visceral projection of sensory nerves changed the neuronal chemistry of the nerve [119], suggesting that the plasticity of afferent neurons is dependent on the peripheral milieu.
Isolating the exact physiological function of each transmitter expressed in vagal afferent neurons requires a multifaceted approach. Firstly, expression of the transmitter needs to be demonstrated in nodose ganglia. Secondly, retrograde tracing experiments must be performed to determine the visceral sites that these neurons innervate. Subsequently the physiological stimuli that regulate gene expression and synthesis of the transmitters must be identified, as well as the peripheral signals that mediate release of the transmitters from terminals in the NTS. Phenotypical changes produced by the release of the endogenous transmitter from vagal afferent fibers into the NTS should be ascertained. Finally, it should be determined if this is a direct or modulatory effect.
While immunohistochemistry and in situ hybridization studies have demonstrated the presence of protein and/or mRNA of an impressive array of transmitters, many of the other necessary studies have not yet been completed to fully understand the physiological role of these transmitters (see Table 1). Nevertheless, there is some evidence that substance P, nitric oxide, and GABA may all be involved in regulating cardiovascular function in response to peripheral signals from the heart. The tachykinin, neurokinin A, has been implicated in respiration in response to peripheral signals from the lungs. Along with the data presented above suggesting a role for CART and MCH in the control of food intake, there is evidence to support the concept of pathway-specific neuropeptide expression in vagal afferent neurons to regulate the varied physiological effects of glutamate.
9. Cardiovascular function
9.1. Substance P
Substance P has been observed in a scattered population of neurons in the rostral end of nodose ganglia [120,121]. Ligation of the vagus nerve causes accumulation of substance P only on the proximal side, suggesting that substance P is transported in vagal afferent fibers from its site of production in the nodose ganglia [120]. Densely labeled substance P terminals in the medial and commissural NTS are significantly reduced following capsaicin treatment [122]. In accordance with this, radioimmunoassay analysis of micropunches from the medial NTS of rats with ganglionectomy showed significantly reduced substance P levels [123].
The primary role of substance P in vagal afferents appears to be in modulating baroreflex control and baroreceptor-mediated blood pressure, though it may also be involved in respiration (see below). Retrogradely labeled studies from carotid sinus and aortic nerve, found a high degree of colocalization of retrogradely labeled nodose ganglia neurons with substance P [124]. Microdialysis experiments found that aortic nerve stimulation in the rat results in substance P release in the NTS [125]. Mechanical activation of baroreceptors in the thoracic aorta that produced baroreflex-dependent decreases in heart rate was accompanied by increases in substance P in the caudal NTS [126].
Three distinct tachykinin receptors exist, neurokinin receptor 1–3 (NK1–3). NK1, which has highest affinity for substance P, is strongly labeled in the medial NTS [127–129]. Vagal afferent fibers appear to be an important source of substance P for NTS neurons, since nodosectomy results in increased NK1 density in the NTS [130]. From extracellular recordings of cardioreceptive NTS neurons, NK1 antagonist CP-99,994 reduced the number of action potentials following electrical stimulation of the vagus nerve [131]. Microinjection of substance P antagonist into the NTS blocks the baseline or reflex effects of substance P [132,133], and ablation of NTS neurons that express NK1 prevents the gain of the baroreflex [134]. Microinjection of substance P into the NTS produced less clear results; in some instances substance P decreased resting blood pressure and heart rate [132,133,135,136], while in other instances substance P had no effect [133,137].
The inconclusive nature of the results produced by microinjection studies of substance P may point to a modulatory role of substance P release from vagal afferent fibers into the NTS. This idea is supported by findings that substance P fibers in the NTS only account for approximately 10% of the axons in the nodose ganglia [120]. Furthermore, substance P appears to potentiate the effects of glutamate in the NTS. Subthreshold doses of substance P microinjected into the NTS of rats increased the depressor response of non-NMDA receptor agonist [138]. Blocking NMDA and non-NMDA receptors in the NTS prevented substance P-induced depressor and bradycardic response [139].
9.2. Nitric oxide
Nitric oxide (NO) is synthesized from l-arginine by nitric oxide synthase (NOS). Nodose ganglia neurons were observed to express the neuronal and endothelial isoforms of NOS (nNOS and eNOS) [140–142]. Fibers in the NTS also contain nNOS [143] and eNOS [142]. nNOS-immunoreactivity in vagal afferent fibers was significantly reduced by nodose ganglionectomy [143–145]. There is evidence that nitric oxide (NO) may participate in cardiovascular signaling from vagal afferent neurons to the NTS. A large proportion of nodose ganglia neurons retrogradely labeled from the aorta depressor nerve colocalize with nNOS [146]. Microinjection of S-nitrosocysteine, an NO donor, into the NTS decreases arterial pressure and heart rate [147,148]. Furthermore, L-NAME, a NOS inhibitor, evokes increases in arterial pressure and heart rate [149].
Extensive colocalization has been observed in fibers of the NTS between nNOS and vGluT2, a marker of glutamate uptake [150]. Importantly similar findings were reported in nodose ganglia neurons retrogradely traced from the aorta depressor nerve [146]. Pharmacological studies further support the idea that glutamate and NO may interact in the NTS to regulate cardiovascular function. L-NMMA, which inhibits the production of NO, significantly reduced depressor but not bradycardic responses elicited by L-glutamate [151]. A separate study found that both the depressor and bradycardic effects of glutamate or NMDA were blocked by prior administration of NO synthase inhibitors L-NMMA or L-NAME [152]. Responses to NMDA microinjected unilater-ally into the NTS of anesthetized rats were significantly attenuated in a dose-dependent manner by prior IP injection of the nNOS inhibitor 7-nitroindazole (7-NI) [153]. L-NAME microinjections into the NTS reduced both AMPA-evoked cardiovascular changes and NMDA-evoked falls in arterial pressure and heart rate [154].
9.3. Gamma-aminobutyric acid
GABA is expressed in a subset of vagal afferent fibers in the NTS, and the number of fibers are reduced in response to sectioning of the vagus nerve [23]. Approximately 20% of the neurons in the nodose ganglia were observed to express GABA [155]. GABA is also expressed by a number of NTS neurons and in fibers projecting from other regions of the brain, so it is difficult to delineate the source of GABA when trying to determine its functional role. However, microinjection of GABA agonists into the NTS increases blood pressure [156], suggesting that were GABA to be released from vagal afferent neurons it may regulate cardiovascular changes.
GABAA and GABAB binding sites are found in the NTS [157] and both receptor types appear to affect blood pressure control. GABAA antagonist enhances responses to aortic depressor nerve stimulation [158,159]. Baclofen, a GABAB antagonist, injected into the NTS, increases blood pressure [160] and attenuates decreased blood pressure following electrical activation of the aortic depressor nerve [161]. Interestingly, evidence for cross-talk between GABA and glutamate has been demonstrated in hippocampal and cortical neurons [162].
10. Respiration
10.1. Neurokinin A
Neurokinin A (NKA) has been found in nodose ganglia neurons [163, 164]. Unilateral nodose ganglionectomy causes a significant decrease in the content of NKA fibers in the NTS of rats [165]. Increased levels of preprotachykinin-A mRNA, which encodes NKA were found in nodose ganglia following 24 h allergen challenge in guinea pigs [166]. NKA microinjection into the NTS reduced respiratory frequency and increased tidal volume [167,168]. Interestingly NK2 receptor, which has high affinity for neurokinin A, but not NK1, which has high affinity for substance P, were found to be involved in the excitatory modulation of inspiratory activity [169]. It is possible that NKA modulates the neuronal response to glutamate in the NTS since NKA has been shown to modulate NMDA and AMPA activation in neurons of the dorsal horn [170]; however this has not properly been tested in the NTS.
Substance P injection into the NTS has also been found to have a role in modulating respiration [171,172]. This is unsurprising seeing both NKA and substance P are products of the same precursor, and both NKA and substance P are colocalized in nodose ganglia neurons. Although NK2 has higher affinity for NKA, substance P is still able to activate NK2 receptors. Furthermore, guinea pigs exposed to second hand smoke for 5 weeks had increased coughing and bronchoconstriction [173], and substance P injected into the NTS has been shown to cause apnea in rats [172]. Therefore substance P may be involved in mediating the overlap in respiration and cardiovascular signaling [72].
11. Conclusion
Glutamate is an important neurotransmitter in visceral afferent neurons of the vagus nerve and is important in mediating a range of physiological functions within the NTS. Here, a model has been proposed to suggest a role for neuropeptide transmitters, expressed by nodose ganglia neurons and released in the NTS, in gating glutamate-induced activation of NTS neurons. There is strong evidence that vagal afferent neurons can integrate signals at least in response to nutritional status to change their neurochemical phenotype. This previously unappreciated plasticity can lead to changes in behavior. A more detailed analysis of the expression patterns of these transmitters and their function may provide novel insights into the pathophysiology of many diseases, especially metabolic syndrome. It is possible that these neuropeptide transmitters may also play important independent roles in coordinating the cross-talk between NTS neurons with different physiological roles to help integrate whole body physiology. This type of research can provide a link between environmental factors and diseases, such as the role of inhalants in obesity. The development of novel conditional knockout mice and viral-based approaches may help to shed some light on the full range of effects of these neuromodulators in nodose ganglia neurons in health and disease.
HIGHLIGHTS.
Role of glutamate in vagal afferent signaling
Problems with glutamate as the only neurotransmitter in vagal afferent neurons
Evidence for existence of neuropeptide transmitters in vagal afferent neurons
Putative role of peptide transmitters in modulating glutamate signaling
Plasticity provided by vagal peptide transmitters in regulating feeding behavior
Acknowledgment
This manuscript is based on work presented during the 2013 Annual Meeting of the Society for the Study of Ingestive Behavior, July 30–August 3, 2013. I would like to thank Prof. Graham Dockray, Dr. Kirsteen Browning, and Dr. Helen Raybould for detailed discussions. Financial support was provided by NIDDK (K99 DK094871).
References
- 1.Ewart WR, Jones MV, King BF. Central origin of vagal nerve fibres innervating the fundus and corpus of the stomach in rat. J Auton Nerv Syst. 1988;25:219–31. doi: 10.1016/0165-1838(88)90026-4. [DOI] [PubMed] [Google Scholar]
- 2.Berthoud HR, Kressel M, Neuhuber WL. An anterograde tracing study of the vagal innervation of rat liver, portal vein and biliary system. Anat Embryol. 1992;186:431–42. doi: 10.1007/BF00185458. [DOI] [PubMed] [Google Scholar]
- 3.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Autonomic neuroscience: basic & clinical. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 4.Broussard DL, Altschuler SM. Brainstem viscerotopic organization of afferents and efferents involved in the control of swallowing. Am J Med. 2000;108(Suppl. 4a):79S–86S. doi: 10.1016/s0002-9343(99)00343-5. [DOI] [PubMed] [Google Scholar]
- 5.Hornby PJ. Receptors and transmission in the brain-gut axis II. Excitatory amino acid receptors in the brain-gut axis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1055–60. doi: 10.1152/ajpgi.2001.280.6.G1055. [DOI] [PubMed] [Google Scholar]
- 6.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng H, Patterson LM, Morrison C, Banfield BW, Randall JA, Browning KN, et al. Melanin concentrating hormone innervation of caudal brainstem areas involved in gastrointestinal functions and energy balance. Neuroscience. 2005;135:611–25. doi: 10.1016/j.neuroscience.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 8.Ritter RC. A tale of two endings: modulation of satiation by NMDA receptors on or near central and peripheral vagal afferent terminals. Physiol Behav. 2011;105:94–9. doi: 10.1016/j.physbeh.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Julio-Pieper M, Flor PJ, Dinan TG, Cryan JF. Exciting times beyond the brain: metabotropic glutamate receptors in peripheral and non-neural tissues. Pharmacol Rev. 2011;63:35–58. doi: 10.1124/pr.110.004036. [DOI] [PubMed] [Google Scholar]
- 10.Kline DD. Plasticity in glutamatergic NTS neurotransmission. Respir Physiol Neurobiol. 2008;164:105–11. doi: 10.1016/j.resp.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawrence AJ. Neurotransmitter mechanisms of rat vagal afferent neurons. Clin Exp Pharmacol Physiol. 1995;22:869–73. doi: 10.1111/j.1440-1681.1995.tb01953.x. [DOI] [PubMed] [Google Scholar]
- 12.Saha S, Batten TF, McWilliam PN. Glutamate-immunoreactivity in identified vagal afferent terminals of the cat: a study combining horseradish peroxidase tracing and postembedding electron microscopic immunogold staining. Exp Physiol. 1995;80:193–202. doi: 10.1113/expphysiol.1995.sp003839. [DOI] [PubMed] [Google Scholar]
- 13.Schaffar N, Rao H, Kessler JP, Jean A. Immunohistochemical detection of glutamate in rat vagal sensory neurons. Brain Res. 1997;778:302–8. doi: 10.1016/s0006-8993(97)01058-5. [DOI] [PubMed] [Google Scholar]
- 14.Talman WT, Perrone MH, Reis DJ. Evidence for L-glutamate as the neurotransmitter of baroreceptor afferent nerve fibers. Science. 1980;209:813–5. doi: 10.1126/science.6105709. [DOI] [PubMed] [Google Scholar]
- 15.Lachamp P, Crest M, Kessler JP. Vesicular glutamate transporters type 1 and 2 expression in axon terminals of the rat nucleus of the solitary tract. Neuroscience. 2006;137:73–81. doi: 10.1016/j.neuroscience.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 16.Corbett EK, Sinfield JK, McWilliam PN, Deuchars J, Batten TF. Differential expression of vesicular glutamate transporters by vagal afferent terminals in rat nucleus of the solitary tract: projections from the heart preferentially express vesicular glutamate transporter 1. Neuroscience. 2005;135:133–45. doi: 10.1016/j.neuroscience.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Cai Y, Hay M, Bishop VS. Synaptic connections and interactions between area postrema and nucleus tractus solitarius. Brain Res. 1996;724:121–4. doi: 10.1016/0006-8993(96)00282-x. [DOI] [PubMed] [Google Scholar]
- 18.Schaffar N, Pio J, Jean A. Selective retrograde labeling of primary vagal afferent cell-bodies after injection of [3H]D-aspartate into the rat nucleus tractus solitarii. Neurosci Lett. 1990;114:253–8. doi: 10.1016/0304-3940(90)90572-q. [DOI] [PubMed] [Google Scholar]
- 19.Granata AR, Sved AF, Reis DJ. In vivo release by vagal stimulation of L-[3H] glutamic acid in the nucleus tractus solitarius preloaded with L-[3H] glutamine. Brain Res Bull. 1984;12:5–9. doi: 10.1016/0361-9230(84)90207-7. [DOI] [PubMed] [Google Scholar]
- 20.Allchin RE, Batten TF, McWilliam PN, Vaughan PF. Electrical stimulation of the vagus increases extracellular glutamate recovered from the nucleus tractus solitarii of the cat by in vivo microdialysis. Exp Physiol. 1994;79:265–8. doi: 10.1113/expphysiol.1994.sp003761. [DOI] [PubMed] [Google Scholar]
- 21.Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci. 2004;24:4709–17. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slattery JA, Page AJ, Dorian CL, Brierley SM, Blackshaw LA. Potentiation of mouse vagal afferent mechanosensitivity by ionotropic and metabotropic glutamate receptors. J Physiol. 2006;577:295–306. doi: 10.1113/jphysiol.2006.117762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietrich WD, Lowry OH, Loewy AD. The distribution of glutamate, GABA and aspar-tate in the nucleus tractus solitarius of the cat. Brain Res. 1982;237:254–60. doi: 10.1016/0006-8993(82)90576-5. [DOI] [PubMed] [Google Scholar]
- 24.Perrone MH. Biochemical evidence that L-glutamate is a neurotransmitter of primary vagal afferent nerve fibers. Brain Res. 1981;230:283–93. doi: 10.1016/0006-8993(81)90407-8. [DOI] [PubMed] [Google Scholar]
- 25.Sved AF, Backes MG. Neuroanatomical evidence that vagal afferent nerves do not possess a high affinity uptake system for glutamate. J Auton Nerv Syst. 1992;38:219–29. doi: 10.1016/0165-1838(92)90033-d. [DOI] [PubMed] [Google Scholar]
- 26.Sved AF. Lack of change in high affinity glutamate uptake in nucleus tractus solitarius following removal of the nodose ganglion. Brain Res Bull. 1986;16:325–9. doi: 10.1016/0361-9230(86)90053-5. [DOI] [PubMed] [Google Scholar]
- 27.Sved AF, Curtis JT. Amino acid neurotransmitters in nucleus tractus solitarius: an in vivo microdialysis study. J Neurochem. 1993;61:2089–98. doi: 10.1111/j.1471-4159.1993.tb07446.x. [DOI] [PubMed] [Google Scholar]
- 28.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 29.Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- 30.Ambalavanar R, Ludlow CL, Wenthold RJ, Tanaka Y, Damirjian M, Petralia RS. Glutamate receptor subunits in the nucleus of the tractus solitarius and other regions of the medulla oblongata in the cat. J Comp Neurol. 1998;402:75–92. [PubMed] [Google Scholar]
- 31.Broussard DL, Wiedner EB, Li X, Altschuler SM. NMDAR1 mRNA expression in the brainstem circuit controlling esophageal peristalsis. Brain Res Mol Brain Res. 1994;27:329–32. doi: 10.1016/0169-328x(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 32.Austgen JR, Fong AY, Foley CM, Mueller PJ, Kline DD, Heesch CM, et al. Expression of Group I metabotropic glutamate receptors on phenotypically different cells within the nucleus of the solitary tract in the rat. Neuroscience. 2009;159:701–16. doi: 10.1016/j.neuroscience.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andresen MC, Yang MY. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol. 1990;259:H1307–11. doi: 10.1152/ajpheart.1990.259.4.H1307. [DOI] [PubMed] [Google Scholar]
- 34.Glaum SR, Miller RJ. Metabotropic glutamate receptors mediate excitatory transmission in the nucleus of the solitary tract. J Neurosci. 1992;12:2251–8. doi: 10.1523/JNEUROSCI.12-06-02251.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon FJ, Leone C. Non-NMDA receptors in the nucleus of the tractus solitarius play the predominant role in mediating aortic baroreceptor reflexes. Brain Res. 1991;568:319–22. doi: 10.1016/0006-8993(91)91418-z. [DOI] [PubMed] [Google Scholar]
- 36.Aylwin ML, Horowitz JM, Bonham AC. NMDA receptors contribute to primary visceral afferent transmission in the nucleus of the solitary tract. J Neurophysiol. 1997;77:2539–48. doi: 10.1152/jn.1997.77.5.2539. [DOI] [PubMed] [Google Scholar]
- 37.Bednar I, Qian M, Qureshi GA, Kallstrom L, Johnson AE, Carrer H, et al. Glutamate inhibits ingestive behaviour. J Neuroendocrinol. 1994;6:403–8. doi: 10.1111/j.1365-2826.1994.tb00600.x. [DOI] [PubMed] [Google Scholar]
- 38.Burns GA, Ritter RC. The non-competitive NMDA antagonist MK-801 increases food intake in rats. Pharmacol Biochem Behav. 1997;56:145–9. doi: 10.1016/S0091-3057(96)00171-2. [DOI] [PubMed] [Google Scholar]
- 39.Burns GA, Fleischmann LG, Ritter RC. MK-801 interferes with nutrient-related signals for satiation. Appetite. 1998;30:1–12. doi: 10.1006/appe.1997.0139. [DOI] [PubMed] [Google Scholar]
- 40.Burns GA, Ritter RC. Visceral afferent participation in delayed satiation following NMDA receptor blockade. Physiol Behav. 1998;65:361–6. doi: 10.1016/s0031-9384(98)00176-0. [DOI] [PubMed] [Google Scholar]
- 41.Covasa M, Ritter RC, Burns GA. Reduction of food intake by intestinal macronutrient infusion is not reversed by NMDA receptor blockade. Am J Physiol Regul Integr Comp Physiol. 2000;278:R345–51. doi: 10.1152/ajpregu.2000.278.2.R345. [DOI] [PubMed] [Google Scholar]
- 42.Covasa M, Ritter RC, Burns GA. NMDA receptor blockade attenuates CCK-induced reduction of real feeding but not sham feeding. Am J Physiol Regul Integr Comp Physiol. 2004;286:R826–31. doi: 10.1152/ajpregu.00570.2003. [DOI] [PubMed] [Google Scholar]
- 43.Berthoud H, Patterson LM, Morales S, Zheng H. Additive satiety-delaying effects of capsaicin-induced visceral deafferentation and NMDA receptor blockade suggest separate pathways. Pharmacol Biochem Behav. 2000;67:371–5. doi: 10.1016/s0091-3057(00)00378-6. [DOI] [PubMed] [Google Scholar]
- 44.Treece BR, Ritter RC, Burns GA. Lesions of the dorsal vagal complex abolish increases in meal size induced by NMDA receptor blockade. Brain Res. 2000;872:37–43. doi: 10.1016/s0006-8993(00)02432-x. [DOI] [PubMed] [Google Scholar]
- 45.Covasa M, Ritter RC, Burns GA. NMDA receptor participation in control of food in-take by the stomach. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1362–8. doi: 10.1152/ajpregu.2000.278.5.R1362. [DOI] [PubMed] [Google Scholar]
- 46.Shinozaki H, Gotoh Y, Ishida M. Selective N-methyl-D-aspartate (NMDA) antagonists increase gastric motility in the rat. Neurosci Lett. 1990;113:56–61. doi: 10.1016/0304-3940(90)90494-t. [DOI] [PubMed] [Google Scholar]
- 47.Covasa M, Hung CY, Ritter RC, Burns GA. Intracerebroventricular administration of MK-801 increases food intake through mechanisms independent of gastric emptying. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1462–7. doi: 10.1152/ajpregu.00471.2004. [DOI] [PubMed] [Google Scholar]
- 48.Treece BR, Covasa M, Ritter RC, Burns GA. Delay in meal termination follows blockade of N-methyl-D-aspartate receptors in the dorsal hindbrain. Brain Res. 1998;810:34–40. doi: 10.1016/s0006-8993(98)00867-1. [DOI] [PubMed] [Google Scholar]
- 49.Guard DB, Swartz TD, Ritter RC, Burns GA, Covasa M. Blockade of hindbrain NMDA receptors containing NR2 subunits increases sucrose intake. Am J Physiol Regul Integr Comp Physiol. 2009;296:R921–8. doi: 10.1152/ajpregu.90456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hung CY, Covasa M, Ritter RC, Burns GA. Hindbrain administration of NMDA receptor antagonist AP-5 increases food intake in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;290:R642–51. doi: 10.1152/ajpregu.00641.2005. [DOI] [PubMed] [Google Scholar]
- 51.Wright J, Campos C, Herzog T, Covasa M, Czaja K, Ritter RC. Reduction of food intake by cholecystokinin requires activation of hindbrain NMDA-type glutamate receptors. Am J Physiol Regul Integr Comp Physiol. 2011;301:R448–55. doi: 10.1152/ajpregu.00026.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gillespie BR, Burns GA, Ritter RC. NMDA channels control meal size via central vagal afferent terminals. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1504–11. doi: 10.1152/ajpregu.00169.2005. [DOI] [PubMed] [Google Scholar]
- 53.Campos CA, Wright JS, Czaja K, Ritter RC. CCK-induced reduction of food intake and hindbrain MAPK signaling are mediated by NMDA receptor activation. Endocrinology. 2012;153:2633–46. doi: 10.1210/en.2012-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balland B, Lachamp P, Kessler JP, Tell F. Silent synapses in developing rat nucleus tractus solitarii have AMPA receptors. J Neurosci. 2008;28:4624–34. doi: 10.1523/JNEUROSCI.5355-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berthoud HR, Earle T, Zheng H, Patterson LM, Phifer C. Food-related gastrointestinal signals activate caudal brainstem neurons expressing both NMDA and AMPA receptors. Brain Res. 2001;915:143–54. doi: 10.1016/s0006-8993(01)02826-8. [DOI] [PubMed] [Google Scholar]
- 56.Mazzone SB, Canning BJ. Central nervous system control of the airways: pharmacological implications. Curr Opin Pharmacol. 2002;2:220–8. doi: 10.1016/s1471-4892(02)00151-0. [DOI] [PubMed] [Google Scholar]
- 57.Machado BH, Mauad H, Chianca Junior DA, Haibara AS, Colombari E. Autonomic processing of the cardiovascular reflexes in the nucleus tractus solitarii. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica. 1997;30:533–43. doi: 10.1590/s0100-879x1997000400015. [et al.] [DOI] [PubMed] [Google Scholar]
- 58.Machado BH. Neurotransmission of the cardiovascular reflexes in the nucleus tractus solitarii of awake rats. Ann N Y Acad Sci. 2001;940:179–96. doi: 10.1111/j.1749-6632.2001.tb03676.x. [DOI] [PubMed] [Google Scholar]
- 59.Smeraski CA, Dunwiddie TV, Diao L, Finger TE. Excitatory amino acid neurotrans-mission in the primary gustatory nucleus of the goldfish Carassius auratus. Ann N Y Acad Sci. 1998;855:442–9. doi: 10.1111/j.1749-6632.1998.tb10604.x. [DOI] [PubMed] [Google Scholar]
- 60.Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130:1007S–15S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- 61.Schwartz NE, Alford S. Physiological activation of presynaptic metabotropic glutamate receptors increases intracellular calcium and glutamate release. J Neurophysiol. 2000;84:415–27. doi: 10.1152/jn.2000.84.1.415. [DOI] [PubMed] [Google Scholar]
- 62.Eulenburg V, Gomeza J. Neurotransmitter transporters expressed in glial cells as regulators of synapse function. Brain Res Rev. 2010;63:103–12. doi: 10.1016/j.brainresrev.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 63.Polson JW, Dampney RA, Boscan P, Pickering AE, Paton JF. Differential baroreflex control of sympathetic drive by angiotensin II in the nucleus tractus solitarii. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1954–60. doi: 10.1152/ajpregu.00041.2007. [DOI] [PubMed] [Google Scholar]
- 64.Altschuler SM, Bao XM, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283:248–68. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- 65.Ciriello J, Schultz CG, Roder S. Collateral axonal projections from ventrolateral medullary non-catecholaminergic neurons to central nucleus of the amygdala. Brain Res. 1994;663:346–51. doi: 10.1016/0006-8993(94)91285-8. [DOI] [PubMed] [Google Scholar]
- 66.Schwarz J, Burguet J, Rampin O, Fromentin G, Andrey P, Tome D, et al. Three-dimensional macronutrient-associated Fos expression patterns in the mouse brainstem. PLoS One. 2010;5:e8974. doi: 10.1371/journal.pone.0008974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paton JF. The Sharpey-Schafer prize lecture: nucleus tractus solitarii: integrating structures. Exp Physiol. 1999;84:815–33. [PubMed] [Google Scholar]
- 68.Bailey TW, Hermes SM, Whittier KL, Aicher SA, Andresen MC. A-type potassium channels differentially tune afferent pathways from rat solitary tract nucleus to caudal ventrolateral medulla or paraventricular hypothalamus. J Physiol. 2007;582:613–28. doi: 10.1113/jphysiol.2007.132365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ruppersberg JPKE, Schwepfer R. The mechanism of magnesium block of NMDA receptors. Semin Neurosci. 1994;6:87–96. [Google Scholar]
- 70.Kullmann DM, Asztely F. Extrasynaptic glutamate spillover in the hippocampus: evidence and implications. Trends Neurosci. 1998;21:8–14. doi: 10.1016/s0166-2236(97)01150-8. [DOI] [PubMed] [Google Scholar]
- 71.Chen CY, Bonham AC. Glutamate suppresses GABA release via presynaptic metabotropic glutamate receptors at baroreceptor neurones in rats. J Physiol. 2005;562:535–51. doi: 10.1113/jphysiol.2004.076885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gilbey MP, Jordan D, Richter DW, Spyer KM. Synaptic mechanisms involved in the inspiratory modulation of vagal cardio-inhibitory neurones in the cat. J Physiol. 1984;356:65–78. doi: 10.1113/jphysiol.1984.sp015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hokfelt T, Broberger C, Xu ZQ, Sergeyev V, Ubink R, Diez M. Neuropeptides–an overview. Neuropharmacology. 2000;39:1337–56. doi: 10.1016/s0028-3908(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 74.Mens WB, Witter A, van Wimersma Greidanus TB. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 1983;262:143–9. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- 75.Fried G, Terenius L, Hokfelt T, Goldstein M. Evidence for differential localization of noradrenaline and neuropeptide Y in neuronal storage vesicles isolated from rat vas deferens. J Neurosci. 1985;5:450–8. doi: 10.1523/JNEUROSCI.05-02-00450.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu PC, Thureson-Klein A, Klein RL. Exocytosis from large dense cored vesicles outside the active synaptic zones of terminals within the trigeminal subnucleus caudalis: a possible mechanism for neuropeptide release. Neuroscience. 1986;19:43–54. doi: 10.1016/0306-4522(86)90004-7. [DOI] [PubMed] [Google Scholar]
- 77.Verhage M, McMahon HT, Ghijsen WE, Boomsma F, Scholten G, Wiegant VM, et al. Differential release of amino acids, neuropeptides, and catecholamines from isolated nerve terminals. Neuron. 1991;6:517–24. doi: 10.1016/0896-6273(91)90054-4. [DOI] [PubMed] [Google Scholar]
- 78.Thureson-Klein A, Klein RL, Zhu PC. Exocytosis from large dense cored vesicles as a mechanism for neuropeptide release in the peripheral and central nervous system. Scan Electron Microsc. 1986:179–87. [PubMed] [Google Scholar]
- 79.Thureson-Klein AK, Klein RL. Exocytosis from neuronal large dense-cored vesicles. Int Rev Cytol. 1990;121:67–126. doi: 10.1016/s0074-7696(08)60659-2. [DOI] [PubMed] [Google Scholar]
- 80.Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J. 2001;20:5887–97. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van de Bospoort R, Farina M, Schmitz SK, de Jong A, de Wit H, Verhage M, et al. Munc13 controls the location and efficiency of dense-core vesicle release in neurons. J Cell Biol. 2012;199:883–91. doi: 10.1083/jcb.201208024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Lartigue G, Dimaline R, Varro A, Dockray GJ. Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J Neurosci. 2007;27:2876–82. doi: 10.1523/JNEUROSCI.5508-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Broberger C, Holmberg K, Kuhar MJ, Hokfelt T. Cocaine- and amphetamine-regulated transcript in the rat vagus nerve: A putative mediator of cholecystokinin-induced satiety. Proc Natl Acad Sci U S A. 1999;96:13506–11. doi: 10.1073/pnas.96.23.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng H, Patterson LM, Berthoud HR. CART in the dorsal vagal complex: sources of immunoreactivity and effects on Fos expression and food intake. Brain Res. 2002;957:298–310. doi: 10.1016/s0006-8993(02)03640-5. [DOI] [PubMed] [Google Scholar]
- 85.Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391:115–32. [PubMed] [Google Scholar]
- 86.De Lartigue G, Dimaline R, Varro A, Raybould H, De la Serre CB, Dockray GJ. Cocaine- and amphetamine-regulated transcript mediates the actions of cholecystokinin on rat vagal afferent neurons. Gastroenterology. 2010;138:1479–90. doi: 10.1053/j.gastro.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Lartigue G, Lur G, Dimaline R, Varro A, Raybould H, Dockray GJ. EGR1 Is a target for cooperative interactions between cholecystokinin and leptin, and inhibition by ghrelin, in vagal afferent neurons. Endocrinology. 2010;151:3589–99. doi: 10.1210/en.2010-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–6. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 89.Vrang N, Tang-Christensen M, Larsen PJ, Kristensen P. Recombinant CART peptide induces c-Fos expression in central areas involved in control of feeding behaviour. Brain Res. 1999;818:499–509. doi: 10.1016/s0006-8993(98)01349-3. [DOI] [PubMed] [Google Scholar]
- 90.Asakawa A, Inui A, Yuzuriha H, Nagata T, Kaga T, Ueno N, et al. Cocaine-amphetamine-regulated transcript influences energy metabolism, anxiety and gastric emptying in mice. Horm Metab Res. 2001;33:554–8. doi: 10.1055/s-2001-17205. [DOI] [PubMed] [Google Scholar]
- 91.Stanley SA, Small CJ, Murphy KG, Rayes E, Abbott CR, Seal LJ, et al. Actions of cocaine- and amphetamine-regulated transcript (CART) peptide on regulation of appetite and hypothalamo-pituitary axes in vitro and in vivo in male rats. Brain Res. 2001;893:186–94. doi: 10.1016/s0006-8993(00)03312-6. [DOI] [PubMed] [Google Scholar]
- 92.Lambert PD, Couceyro PR, McGirr KM, Dall Vechia SE, Smith Y, Kuhar MJ. CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse. 1998;29:293–8. doi: 10.1002/(SICI)1098-2396(199808)29:4<293::AID-SYN1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 93.Okumura T, Yamada H, Motomura W, Kohgo Y. Cocaine-amphetamine-regulated transcript (CART) acts in the central nervous system to inhibit gastric acid secretion via brain corticotropin-releasing factor system. Endocrinology. 2000;141:2854–60. doi: 10.1210/endo.141.8.7588. [DOI] [PubMed] [Google Scholar]
- 94.Tebbe JJ, Ortmann E, Schumacher K, Monnikes H, Kobelt P, Arnold R, et al. Cocaine-and amphetamine-regulated transcript stimulates colonic motility via central CRF receptor activation and peripheral cholinergic pathways in fed, conscious rats. Neurogastroenterol Motil. 2004;16:489–96. doi: 10.1111/j.1365-2982.2004.00561.x. [DOI] [PubMed] [Google Scholar]
- 95.Zheng H, Patterson C, Berthoud HR. Fourth ventricular injection of CART peptide inhibits short-term sucrose intake in rats. Brain Res. 2001;896:153–6. doi: 10.1016/s0006-8993(00)03256-x. [DOI] [PubMed] [Google Scholar]
- 96.Aja S, Robinson BM, Mills KJ, Ladenheim EE, Moran TH. Fourth ventricular CART reduces food and water intake and produces a conditioned taste aversion in rats. Behav Neurosci. 2002;116:918–21. [PubMed] [Google Scholar]
- 97.Aja S, Sahandy S, Ladenheim EE, Schwartz GJ, Moran TH. Intracerebroventricular CART peptide reduces food intake and alters motor behavior at a hindbrain site. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1862–7. doi: 10.1152/ajpregu.2001.281.6.R1862. [DOI] [PubMed] [Google Scholar]
- 98.de Lartigue GDRA Raybould HE. Neuropeptide transmitters released from VAN into the NTS play an important role in regulating food intake. Appetite. 2011;57:S12–3. [Google Scholar]
- 99.de Lartigue G. Cocaine- and amphetamine-regulated transcript (CART) derived from vagal afferent neurons is required for satiation. Neurogastroenterol Motility. 2013;251 [Google Scholar]
- 100.Heldsinger A, Lu Y, Zhou SY, Wu X, Grabauskas G, Song I, et al. Cocaine- and amphetamine-regulated transcript is the neurotransmitter regulating the action of cholecystokinin and leptin on short-term satiety in rats. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1042–51. doi: 10.1152/ajpgi.00231.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101.Hsun Lin H, Chiu HY, Lai CC. Potentiation of spinal N-methyl-D-aspartate-mediated nociceptive transmission by cocaine-regulated and amphetamine-regulated transcript peptide in rats. Neuroreport. 2005;16:253–7. doi: 10.1097/00001756-200502280-00010. [DOI] [PubMed] [Google Scholar]
- 102.Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Feeding-dependent depression of melanin-concentrating hormone and melanin-concentrating hormone receptor-1 expression in vagal afferent neurones. Neuroscience. 2006;137:1405–15. doi: 10.1016/j.neuroscience.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 103.Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1289–97. doi: 10.1152/ajpgi.00543.2005. [DOI] [PubMed] [Google Scholar]
- 104.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–7. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 105.Nair SG, Adams-Deutsch T, Pickens CL, Smith DG, Shaham Y. Effects of the MCH1 receptor antagonist SNAP 94847 on high-fat food-reinforced operant responding and reinstatement of food seeking in rats. Psychopharmacology (Berl) 2009;205:129–40. doi: 10.1007/s00213-009-1523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Segal-Lieberman G, Bradley RL, Kokkotou E, Carlson M, Trombly DJ, Wang X, et al. Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc Natl Acad Sci U S A. 2003;100:10085–90. doi: 10.1073/pnas.1633636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen Y, Hu C, Hsu CK, Zhang Q, Bi C, Asnicar M, et al. Targeted disruption of the melanin-concentrating hormone receptor-1 results in hyperphagia and resistance to diet-induced obesity. Endocrinology. 2002;143:2469–77. doi: 10.1210/endo.143.7.8903. [DOI] [PubMed] [Google Scholar]
- 108.Marsh DJ, Weingarth DT, Novi DE, Chen HY, Trumbauer ME, Chen AS, et al. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci U S A. 2002;99:3240–5. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, et al. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–45. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- 110.Hervieu GJ, Cluderay JE, Harrison D, Meakin J, Maycox P, Nasir S, et al. The distribution of the mRNA and protein products of the melanin-concentrating hormone (MCH) receptor gene, slc-1, in the central nervous system of the rat. Eur J Neurosci. 2000;12:1194–216. doi: 10.1046/j.1460-9568.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- 111.Skofitsch G, Jacobowitz DM, Zamir N. Immunohistochemical localization of a melanin concentrating hormone-like peptide in the rat brain. Brain Res Bull. 1985;15:635–49. doi: 10.1016/0361-9230(85)90213-8. [DOI] [PubMed] [Google Scholar]
- 112.Zamir N, Skofitsch G, Jacobowitz DM. Distribution of immunoreactive melanin-concentrating hormone in the central nervous system of the rat. Brain Res. 1986;373:240–5. doi: 10.1016/0006-8993(86)90337-9. [DOI] [PubMed] [Google Scholar]
- 113.Zamir N, Skofitsch G, Bannon MJ, Jacobowitz DM. Melanin-concentrating hormone: unique peptide neuronal system in the rat brain and pituitary gland. Proc Natl Acad Sci U S A. 1986;83:1528–31. doi: 10.1073/pnas.83.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bresson JL, Clavequin MC, Fellmann D, Bugnon C. Human hypothalamic neuronal system revealed with a salmon melanin-concentrating hormone (MCH) antiserum. Neurosci Lett. 1989;102:39–43. doi: 10.1016/0304-3940(89)90304-2. [DOI] [PubMed] [Google Scholar]
- 115.Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons: kinetics and role in influencing neurochemical phenotype. Am J Physiol Gastrointest Liver Physiol. 2010;299:G63–9. doi: 10.1152/ajpgi.00059.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vallee RB, Bloom GS. Mechanisms of fast and slow axonal transport. Annu Rev Neurosci. 1991;14:59–92. doi: 10.1146/annurev.ne.14.030191.000423. [DOI] [PubMed] [Google Scholar]
- 117.Hammerschlag R, Stone GC, Bolen FA, Lindsey JD, Ellisman MH. Evidence that all newly synthesized proteins destined for fast axonal transport pass through the Golgi apparatus. J Cell Biol. 1982;93:568–75. doi: 10.1083/jcb.93.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gibbins IL, Furness JB, Costa M. Pathway-specific patterns of the co-existence of substance P, calcitonin gene-related peptide, cholecystokinin and dynorphin in neurons of the dorsal root ganglia of the guinea-pig. Cell Tissue Res. 1987;248:417–37. doi: 10.1007/BF00218210. [DOI] [PubMed] [Google Scholar]
- 119.McMahon SB, Gibson S. Peptide expression is altered when afferent nerves reinnervate inappropriate tissue. Neurosci Lett. 1987;73:9–15. doi: 10.1016/0304-3940(87)90022-x. [DOI] [PubMed] [Google Scholar]
- 120.Gamse R, Lembeck F, Cuello AC. Substance P in the vagus nerve Immunochemical and immunohistochemical evidence for axoplasmic transport. Naunyn Schmiedebergs Arch Pharmacol. 1979;306:37–44. doi: 10.1007/BF00515591. [DOI] [PubMed] [Google Scholar]
- 121.Lundberg JM, Hokfelt T, Nilsson G, Terenius L, Rehfeld J, Elde R, et al. Peptide neurons in the vagus, splanchnic and sciatic nerves. Acta Physiol Scand. 1978;104:499–501. doi: 10.1111/j.1748-1716.1978.tb06307.x. [DOI] [PubMed] [Google Scholar]
- 122.Lorez HP, Haeusler G, Aeppli L. Substance P neurones in medullary baroreflex areas and baroreflex function of capsaicin-treated rats Comparison with other primary afferent systems. Neuroscience. 1983;8:507–23. doi: 10.1016/0306-4522(83)90196-3. [DOI] [PubMed] [Google Scholar]
- 123.Gillis RA, Helke CJ, Hamilton BL, Norman WP, Jacobowitz DM. Evidence that substance P is a neurotransmitter of baro- and chemoreceptor afferents in nucleus tractus solitarius. Brain Res. 1980;181:476–81. doi: 10.1016/0006-8993(80)90633-2. [DOI] [PubMed] [Google Scholar]
- 124.Helke CJ, Goldman W, Jacobowitz DM. Demonstration of substance P in aortic nerve afferent fibers by combined use of fluorescent retrograde neuronal labeling and immunocytochemistry. Peptides. 1980;1:359–64. doi: 10.1016/0196-9781(80)90015-7. [DOI] [PubMed] [Google Scholar]
- 125.Morilak DA, Morris M, Chalmers J. Release of substance P in the nucleus tractus solitarius measured by in vivo microdialysis: response to stimulation of the aortic depressor nerves in rabbit. Neurosci Lett. 1988;94:131–7. doi: 10.1016/0304-3940(88)90283-2. [DOI] [PubMed] [Google Scholar]
- 126.Potts JT, Fuchs IE. Naturalistic activation of barosensitive afferents release substance P in the nucleus tractus solitarius of the cat. Brain Res. 2001;893:155–64. doi: 10.1016/s0006-8993(00)03308-4. [DOI] [PubMed] [Google Scholar]
- 127.Danks JA, Rothman RB, Cascieri MA, Chicchi GG, Liang T, Herkenham M. A comparative autoradiographic study of the distributions of substance P and eledoisin binding sites in rat brain. Brain Res. 1986;385:273–81. doi: 10.1016/0006-8993(86)91073-5. [DOI] [PubMed] [Google Scholar]
- 128.Helke CJ, Shults CW, Chase TN, O'Donohue TL. Autoradiographic localization of substance P receptors in rat medulla: effect of vagotomy and nodose ganglionectomy. Neuroscience. 1984;12:215–23. doi: 10.1016/0306-4522(84)90148-9. [DOI] [PubMed] [Google Scholar]
- 129.Maley BE, Sasek CA, Seybold VS. Substance P binding sites in the nucleus tractus solitarius of the cat. Peptides. 1988;9:1301–6. doi: 10.1016/0196-9781(88)90196-9. [DOI] [PubMed] [Google Scholar]
- 130.Segu L, Lanoir J, Puizillout JJ. Up-regulation of substance P binding sites in the vagus nerve projection area of the cat brainstem after nodosectomy. A quantitative auto-radiographic study. J Chem Neuroanat. 1991;4:447–59. doi: 10.1016/0891-0618(91)90025-8. [DOI] [PubMed] [Google Scholar]
- 131.Paton JF. Importance of neurokinin-1 receptors in the nucleus tractus solitarii of mice for the integration of cardiac vagal inputs. Eur J Neurosci. 1998;10:2261–75. doi: 10.1046/j.1460-9568.1998.00238.x. [DOI] [PubMed] [Google Scholar]
- 132.Chan JY, Barnes CD, Chan SH. Tonic enhancement of the sensitivity of baroreceptor reflex response by endogenous substance P in the rat. Regul Pept. 1990;29:199–213. doi: 10.1016/0167-0115(90)90083-9. [DOI] [PubMed] [Google Scholar]
- 133.Kubo T, Kihara M. Blood pressure modulation by substance P in the rat nucleus tractus solitarius. Brain Res. 1987;413:379–83. doi: 10.1016/0006-8993(87)91033-x. [DOI] [PubMed] [Google Scholar]
- 134.Riley J, Lin LH, Chianca DA, Jr, Talman WT. Ablation of NK1 receptors in rat nucleus tractus solitarii blocks baroreflexes. Hypertension. 2002;40:823–6. doi: 10.1161/01.hyp.0000042089.34004.cf. [DOI] [PubMed] [Google Scholar]
- 135.Hall ME, Miley FB, Stewart JM. Cardiovascular effects of substance P peptides in the nucleus of the solitary tract. Brain Res. 1989;497:280–90. doi: 10.1016/0006-8993(89)90273-4. [DOI] [PubMed] [Google Scholar]
- 136.Haeusler G, Osterwalder R. Evidence suggesting a transmitter or neuromodulatory role for substance P at the first synapse of the baroreceptor reflex. Naunyn Schmiedebergs Arch Pharmacol. 1980;314:111–21. doi: 10.1007/BF00504526. [DOI] [PubMed] [Google Scholar]
- 137.Talman WT, Reis DJ. Baroreflex actions of substance P microinjected into the nucleus tractus solitarii in rat: a consequence of local distortion. Brain Res. 1981;220:402–7. doi: 10.1016/0006-8993(81)91233-6. [DOI] [PubMed] [Google Scholar]
- 138.Cowan AR, Dean C, Bago M, Seagard JL. Potentiation of non-N-methyl-D-aspartate receptor-induced changes in blood pressure by substance P in rats. Neurosci Lett. 2000;278:161–4. doi: 10.1016/s0304-3940(99)00920-9. [DOI] [PubMed] [Google Scholar]
- 139.Hall ME, Greer RA, Stewart JM. Effects of L-glutamate, substance P and substance P(1-7) on cardiovascular regulation in the nucleus tractus solitarius. Regul Pept. 1993;46:102–9. doi: 10.1016/0167-0115(93)90019-5. [DOI] [PubMed] [Google Scholar]
- 140.Nozaki K, Moskowitz MA, Maynard KI, Koketsu N, Dawson TM, Bredt DS, et al. Possible origins and distribution of immunoreactive nitric oxide synthase-containing nerve fibers in cerebral arteries. J Cereb Blood Flow Metab. 1993;13:70–9. doi: 10.1038/jcbfm.1993.9. [DOI] [PubMed] [Google Scholar]
- 141.Lawrence AJ, Jarrott B. Neurochemical modulation of cardiovascular control in the nucleus tractus solitarius. Prog Neurobiol. 1996;48:21–53. doi: 10.1016/0301-0082(95)00034-8. [DOI] [PubMed] [Google Scholar]
- 142.Yamamoto Y, Henrich M, Snipes RL, Kummer W. Altered production of nitric oxide and reactive oxygen species in rat nodose ganglion neurons during acute hypoxia. Brain Res. 2003;961:1–9. doi: 10.1016/s0006-8993(02)03826-x. [DOI] [PubMed] [Google Scholar]
- 143.Ruggiero DA, Mtui EP, Otake K, Anwar M. Central and primary visceral afferents to nucleus tractus solitarii may generate nitric oxide as a membrane-permeant neuronal messenger. J Comp Neurol. 1996;364:51–67. doi: 10.1002/(SICI)1096-9861(19960101)364:1<51::AID-CNE5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 144.Lin LH, Cassell MD, Sandra A, Talman WT. Direct evidence for nitric oxide synthase in vagal afferents to the nucleus tractus solitarii. Neuroscience. 1998;84:549–58. doi: 10.1016/s0306-4522(97)00501-0. [DOI] [PubMed] [Google Scholar]
- 145.Lawrence AJ, Castillo-Melendez M, McLean KJ, Jarrott B. The distribution of nitric oxide synthase-, adenosine deaminase- and neuropeptide Y-immunoreactivity through the entire rat nucleus tractus solitarius: Effect of unilateral nodose ganglionectomy. J Chem Neuroanat. 1998;15:27–40. doi: 10.1016/s0891-0618(98)00020-9. [DOI] [PubMed] [Google Scholar]
- 146.Lin LH, Talman WT. Vesicular glutamate transporters and neuronal nitric oxide synthase colocalize in aortic depressor afferent neurons. J Chem Neuroanat. 2006;32:54–64. doi: 10.1016/j.jchemneu.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 147.Lewis SJ, Ohta H, Machado B, Bates JN, Talman WT. Microinjection of S-nitrosocysteine into the nucleus tractus solitarii decreases arterial pressure and heart rate via activation of soluble guanylate cyclase. Eur J Pharmacol. 1991;202:135–6. doi: 10.1016/0014-2999(91)90269-v. [DOI] [PubMed] [Google Scholar]
- 148.Machado BH, Bonagamba LG. Microinjection of S-nitrosocysteine into the nucleus tractus solitarii of conscious rats decreases arterial pressure but L-glutamate does not. Eur J Pharmacol. 1992;221:179–82. doi: 10.1016/0014-2999(92)90791-2. [DOI] [PubMed] [Google Scholar]
- 149.Harada S, Tokunaga S, Momohara M, Masaki H, Tagawa T, Imaizumi T, et al. Inhibition of nitric oxide formation in the nucleus tractus solitarius increases renal sympathetic nerve activity in rabbits. Circ Res. 1993;72:511–6. doi: 10.1161/01.res.72.3.511. [DOI] [PubMed] [Google Scholar]
- 150.Lin LH, Edwards RH, Fremeau RT, Fujiyama F, Kaneko T, Talman WT. Localization of vesicular glutamate transporters and neuronal nitric oxide synthase in rat nucleus tractus solitarii. Neuroscience. 2004;123:247–55. doi: 10.1016/j.neuroscience.2003.08.063. [DOI] [PubMed] [Google Scholar]
- 151.Di Paola ED, Nistico MJV,G. L-Glutamate evokes the release of an endothelium-derived relaxing factor-like substance from the rat nucleus tractus solitarius. J Cardiovasc Pharmacol. 1991;17:S269–72. [Google Scholar]
- 152.Lo WC, Lin HC, Ger LP, Tung CS, Tseng CJ. Cardiovascular effects of nitric oxide and N-methyl-D-aspartate receptors in the nucleus tractus solitarii of rats. Hypertension. 1997;30:1499–503. doi: 10.1161/01.hyp.30.6.1499. [DOI] [PubMed] [Google Scholar]
- 153.Talman WT, Dragon DN, Ohta H, Lin LH. Nitroxidergic influences on cardiovascular control by NTS: A link with glutamate. Ann N Y Acad Sci. 2001;940:169–78. doi: 10.1111/j.1749-6632.2001.tb03675.x. [DOI] [PubMed] [Google Scholar]
- 154.Dias ACR, Colombari E, Mifflin SW. Effect of nitric oxide on excitatory amino acid-evoked discharge of neurons in NTS. Am J Physiol Heart C. 2003;284:H234–40. doi: 10.1152/ajpheart.00037.2002. [DOI] [PubMed] [Google Scholar]
- 155.Stoyanova II. Gamma-aminobutiric acid immunostaining in trigeminal, nodose and spinal ganglia of the cat. Acta Histochem. 2004;106:309–14. doi: 10.1016/j.acthis.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 156.Catelli JM, Giakas WJ, Sved AF. GABAergic mechanisms in nucleus tractus solitarius alter blood pressure and vasopressin release. Brain Res. 1987;403:279–89. doi: 10.1016/0006-8993(87)90065-5. [DOI] [PubMed] [Google Scholar]
- 157.van Giersbergen PL, Palkovits M, De Jong W. Involvement of neurotransmitters in the nucleus tractus solitarii in cardiovascular regulation. Physiol Rev. 1992;72:789–824. doi: 10.1152/physrev.1992.72.3.789. [DOI] [PubMed] [Google Scholar]
- 158.Kubo T, Kihara M. Evidence for gamma-aminobutyric acid receptor-mediated modulation of the aortic baroreceptor reflex in the nucleus tractus solitarii of the rat. Neurosci Lett. 1988;89:156–60. doi: 10.1016/0304-3940(88)90373-4. [DOI] [PubMed] [Google Scholar]
- 159.Bousquet P, Feldman J, Bloch R, Schwartz J. Evidence for a neuromodulatory role of GABA at the first synapse of the baroreceptor reflex pathway Effects of GABA derivatives injected into the NTS. Naunyn Schmiedebergs Arch Pharmacol. 1982;319:168–71. doi: 10.1007/BF00503932. [DOI] [PubMed] [Google Scholar]
- 160.Persson B. A hypertensive response to baclofen in the nucleus tractus solitarii in rats. J Pharm Pharmacol. 1981;33:226–31. doi: 10.1111/j.2042-7158.1981.tb13763.x. [DOI] [PubMed] [Google Scholar]
- 161.Sved AF, Tsukamoto K. Tonic stimulation of GABAB receptors in the nucleus tractus solitarius modulates the baroreceptor reflex. Brain Res. 1992;592:37–43. doi: 10.1016/0006-8993(92)91655-x. [DOI] [PubMed] [Google Scholar]
- 162.Vargas KJ, Terunuma M, Tello JA, Pangalos MN, Moss SJ, Couve A. The availability of surface GABA B receptors is independent of gamma-aminobutyric acid but controlled by glutamate in central neurons. J Biol Chem. 2008;283:24641–8. doi: 10.1074/jbc.M802419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Helke CJ, Hill KM. Immunohistochemical study of neuropeptides in vagal and glossopharyngeal afferent neurons in the rat. Neuroscience. 1988;26:539–51. doi: 10.1016/0306-4522(88)90166-2. [DOI] [PubMed] [Google Scholar]
- 164.Helke CJ, Niederer AJ. Studies on the coexistence of substance P with other putative transmitters in the nodose and petrosal ganglia. Synapse. 1990;5:144–51. doi: 10.1002/syn.890050209. [DOI] [PubMed] [Google Scholar]
- 165.Nagashima A, Takano Y, Tateishi K, Matsuoka Y, Hamaoka T, Kamiya H. Cardiovascular roles of tachykinin peptides in the nucleus tractus solitarii of rats. Brain Res. 1989;487:392–6. doi: 10.1016/0006-8993(89)90848-2. [DOI] [PubMed] [Google Scholar]
- 166.Fischer A, McGregor GP, Saria A, Philippin B, Kummer W. Induction of tachykinin gene and peptide expression in guinea pig nodose primary afferent neurons by allergic airway inflammation. J Clin Invest. 1996;98:2284–91. doi: 10.1172/JCI119039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mazzone SB, Geraghty DP. Respiratory actions of tachykinins in the nucleus of the solitary tract: effect of neonatal capsaicin pretreatment. Br J Pharmacol. 2000;129:1132–9. doi: 10.1038/sj.bjp.0703173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mazzone SB, Geraghty DP. Respiratory actions of tachykinins in the nucleus of the solitary tract: characterization of receptors using selective agonists and antagonists. Br J Pharmacol. 2000;129:1121–31. doi: 10.1038/sj.bjp.0703172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bongianni F, Mutolo D, Cinelli E, Pantaleo T. Neurokinin receptor modulation of respiratory activity in the rabbit. Eur J Neurosci. 2008;27:3233–43. doi: 10.1111/j.1460-9568.2008.06295.x. [DOI] [PubMed] [Google Scholar]
- 170.Rusin KI, Jiang MC, Cerne R, Randic M. Interactions between excitatory amino acids and tachykinins in the rat spinal dorsal horn. Brain Res Bull. 1993;30:329–38. doi: 10.1016/0361-9230(93)90261-9. [DOI] [PubMed] [Google Scholar]
- 171.Joad JP, Sekizawa S, Chen CY, Bonham AC. Air pollutants and cough. Pulm Pharmacol Ther. 2007;20:347–54. doi: 10.1016/j.pupt.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 172.Bauman NM, Wang D, Luschei ES, Talman WT. Effect of substance P injection into the nucleus tractus solitarius of rats on cricothyroid and thyroarytenoid motor activity and cardiovascular and respiratory systems. Ann Otol Rhinol Laryngol. 2002;111:875–83. doi: 10.1177/000348940211101003. [DOI] [PubMed] [Google Scholar]
- 173.Joad JP, Munch PA, Bric JM, Evans SJ, Pinkerton KE, Chen CY, et al. Passive smoke effects on cough and airways in young guinea pigs: role of brainstem substance P. Am J Respir Crit Care Med. 2004;169:499–504. doi: 10.1164/rccm.200308-1139OC. [DOI] [PubMed] [Google Scholar]
- 174.Mazzone SB, McGovern AE. Immunohistochemical characterization of nodose cough receptor neurons projecting to the trachea of guinea pigs. Cough. 2008;4:9. doi: 10.1186/1745-9974-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mascarucci P, Perego C, Terrazzino S, De Simoni MG. Glutamate release in the nucleus tractus solitarius induced by peripheral lipopolysaccharide and interleukin-1 beta. Neuroscience. 1998;86:1285–90. doi: 10.1016/s0306-4522(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 176.Furukawa N, Hatano M, Fukuda H, Koga T. Non-N-methyl-D-aspartate receptors may mediate the transmission of emetic signals between visceral vagal afferents and the solitary nucleus in dogs. Neurosci Lett. 1998;258:53–6. doi: 10.1016/s0304-3940(98)00859-3. [DOI] [PubMed] [Google Scholar]
- 177.Page AJ O'Donnell TA, Cooper NJ, Young RL, Blackshaw LA. Nitric oxide as an endogenous peripheral modulator of visceral sensory neuronal function. J Neurosci. 2009;29:7246–55. doi: 10.1523/JNEUROSCI.6099-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Palouzier B, Barrit-Chamoin MC, Portalier P, Ternaux JP. Cholinergic neurons in the rat nodose ganglia. Neurosci Lett. 1987;80:147–52. doi: 10.1016/0304-3940(87)90644-6. [DOI] [PubMed] [Google Scholar]
- 179.Ternaux JP, Falempin M, Palouzier B, Chamoin MC, Portalier P. Presence of cholinergic neurons in the vagal afferent system: biochemical and immunohistochemical approaches. J Auton Nerv Syst. 1989;28:233–42. doi: 10.1016/0165-1838(89)90151-3. [DOI] [PubMed] [Google Scholar]
- 180.Cheng PW, Lu PJ, Chen SR, Ho WY, Cheng WH, Hong LZ, et al. Central nicotinic acetylcholine receptor involved in Ca(2+) -calmodulin-endothelial nitric oxide synthase pathway modulated hypotensive effects. Br J Pharmacol. 2011;163:1203–13. doi: 10.1111/j.1476-5381.2010.01124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Giacobini E, Nore B. Dopa-decarboxylase in autonomic and sensory ganglia of the cat. Acta Physiol Scand. 1971;82:209–17. doi: 10.1111/j.1748-1716.1971.tb04960.x. [DOI] [PubMed] [Google Scholar]
- 182.Katz DM, Markey KA, Goldstein M, Black IB. Expression of catecholaminergic characteristics by primary sensory neurons in the normal adult rat in vivo. Proc Natl Acad Sci U S A. 1983;80:3526–30. doi: 10.1073/pnas.80.11.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kummer W, Bachmann S, Neuhuber WL, Hanze J, Lang RE. Tyrosine-hydroxylase-containing vagal afferent neurons in the rat nodose ganglion are independent from neuropeptide-Y-containing populations and project to esophagus and stomach. Cell Tissue Res. 1993;271:135–44. doi: 10.1007/BF00297551. [DOI] [PubMed] [Google Scholar]
- 184.Granata AR, Woodruff GN. Dopaminergic mechanisms in the nucleus tractus solitarius and effects on blood pressure. Brain Res Bull. 1982;8:483–8. doi: 10.1016/0361-9230(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 185.Gaudin-Chazal G, Portalier P, Barrit MC, Puizillout JJ. Serotonin-like immunoreactivity in paraffin-sections of the nodose ganglia of the cat. Neurosci Lett. 1982;33:169–72. doi: 10.1016/0304-3940(82)90246-4. [DOI] [PubMed] [Google Scholar]
- 186.Thor KB, Hill KM, Harrod C, Helke CJ. Immunohistochemical and biochemical analysis of serotonin and substance P colocalization in the nucleus tractus solitarii and associated afferent ganglia of the rat. Synapse. 1988;2:225–31. doi: 10.1002/syn.890020309. [DOI] [PubMed] [Google Scholar]
- 187.Nosjean A, Compoint C, Buisseret-Delmas C, Orer HS, Merahi N, Puizillout JJ, et al. Serotonergic projections from the nodose ganglia to the nucleus tractus solitarius: an immunohistochemical and double labeling study in the rat. Neurosci Lett. 1990;114:22–6. doi: 10.1016/0304-3940(90)90422-6. [DOI] [PubMed] [Google Scholar]
- 188.Itoh H, Bunag RD. Cardiovascular and sympathetic effects of injecting serotonin into the nucleus tractus solitarius in rats. J Pharmacol Exp Ther. 1991;256:1147–53. [PubMed] [Google Scholar]
- 189.Voss MD, De Castro D, Lipski J, Pilowsky PM, Jiang C. Serotonin immunoreactive boutons form close appositions with respiratory neurons of the dorsal respiratory group in the cat. J Comp Neurol. 1990;295:208–18. doi: 10.1002/cne.902950205. [DOI] [PubMed] [Google Scholar]
- 190.Czyzyk-Krzeska MF, Bayliss DA, Seroogy KB, Millhorn DE. Gene expression for peptides in neurons of the petrosal and nodose ganglia in rat Experimental brain research. Exp Hirnforsch Exp Cereb. 1991;83:411–8. doi: 10.1007/BF00231166. [DOI] [PubMed] [Google Scholar]
- 191.Kummer W, Fischer A, Kurkowski R, Heym C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience. 1992;49:715–37. doi: 10.1016/0306-4522(92)90239-x. [DOI] [PubMed] [Google Scholar]
- 192.Franco-Cereceda A, Henke H, Lundberg JM, Petermann JB, Hokfelt T, Fischer JA. Calcitonin gene-related peptide (CGRP) in capsaicin-sensitive substance P-immunoreactive sensory neurons in animals and man: distribution and release by capsaicin. Peptides. 1987;8:399–410. doi: 10.1016/0196-9781(87)90117-3. [DOI] [PubMed] [Google Scholar]
- 193.Torrealba F. Calcitonin gene-related peptide immunoreactivity in the nucleus of the tractus solitarius and the carotid receptors of the cat originates from peripheral afferents. Neuroscience. 1992;47:165–73. doi: 10.1016/0306-4522(92)90129-p. [DOI] [PubMed] [Google Scholar]
- 194.Springall DR, Cadieux A, Oliveira H, Su H, Royston D, Polak JM. Retrograde tracing shows that CGRP-immunoreactive nerves of rat trachea and lung originate from vagal and dorsal root ganglia. J Auton Nerv Syst. 1987;20:155–66. doi: 10.1016/0165-1838(87)90113-5. [DOI] [PubMed] [Google Scholar]
- 195.Uddman R, Grunditz T, Luts A, Desai H, Fernstrom G, Sundler F. Distribution and origin of the peripheral innervation of rat cervical esophagus. Dysphagia. 1995;10:203–12. doi: 10.1007/BF00260977. [DOI] [PubMed] [Google Scholar]
- 196.Lutz TA, Senn M, Althaus J, Del Prete E, Ehrensperger F, Scharrer E. Lesion of the area postrema/nucleus of the solitary tract (AP/NTS) attenuates the anorectic effects of amylin and calcitonin gene-related peptide (CGRP) in rats. Peptides. 1998;19:309–17. doi: 10.1016/s0196-9781(97)00292-1. [DOI] [PubMed] [Google Scholar]
- 197.Lundberg JM, Dahlstrom A, Larsson I, Petterson G, Ahlman H, Kewenter J. Efferent innervation of the small intestine by adrenergic neurons from the cervical sympathetic and stellate ganglia, studied by retrograde transport of peroxidase. Acta Physiol Scand. 1978;104:33–42. doi: 10.1111/j.1748-1716.1978.tb06248.x. [DOI] [PubMed] [Google Scholar]
- 198.Dockray GJ, Gregory RA, Tracy HJ, Zhu WY. Transport of cholecystokininoctapeptide-like immunoreactivity toward the gut in afferent vagal fibres in cat and dog. J Physiol. 1981;314:501–11. doi: 10.1113/jphysiol.1981.sp013721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mantyh PW, Hunt SP. Neuropeptides are present in projection neurones at all levels in visceral and taste pathways: from periphery to sensory cortex. Brain Res. 1984;299:297–312. doi: 10.1016/0006-8993(84)90711-x. [DOI] [PubMed] [Google Scholar]
- 200.Broberger C, Holmberg K, Shi TJ, Dockray G, Hokfelt T. Expression and regulation of cholecystokinin and cholecystokinin receptors in rat nodose and dorsal root ganglia. Brain Res. 2001;903:128–40. doi: 10.1016/s0006-8993(01)02468-4. [DOI] [PubMed] [Google Scholar]
- 201.Philippe C, Cuber JC, Bosshard A, Rampin O, Laplace JP, Chayvialle JA. Galanin in porcine vagal sensory nerves: immunohistochemical and immunochemical study. Peptides. 1990;11:989–93. doi: 10.1016/0196-9781(90)90022-w. [DOI] [PubMed] [Google Scholar]
- 202.Calingasan NY, Ritter S. Presence of galanin in rat vagal sensory neurons: evidence from immunohistochemistry and in situ hybridization. J Auton Nerv Syst. 1992;40:229–38. doi: 10.1016/0165-1838(92)90205-u. [DOI] [PubMed] [Google Scholar]
- 203.Page AJ, Slattery JA, O'Donnell T,A, Cooper NJ, Young RL, Blackshaw LA. Modulation of gastro-oesophageal vagal afferents by galanin in mouse and ferret. J Physiol. 2005;563:809–19. doi: 10.1113/jphysiol.2004.075291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Koegler FH, Ritter S. Galanin injection into the nucleus of the solitary tract stimulates feeding in rats with lesions of the paraventricular nucleus of the hypothalamus. Physiol Behav. 1998;63:521–7. doi: 10.1016/s0031-9384(97)00480-0. [DOI] [PubMed] [Google Scholar]
- 205.Harfstrand A, Fuxe K, Melander T, Hokfelt T, Agnati LF. Evidence for a cardiovascular role of central galanin neurons: focus on interactions with alpha 2-adrenergic and neuropeptide Y mechanisms. J Cardiovasc Pharmacol. 1987;10(Suppl. 12):S199–204. [PubMed] [Google Scholar]
- 206.Ferrari MF, Coelho EF, Farizatto KL, Chadi G, Fior-Chadi DR. Modulation of tyrosine hydroxylase, neuropeptide y, glutamate, and substance p in Ganglia and brain areas involved in cardiovascular control after chronic exposure to nicotine. Int J Hypertens. 2011;2011:216464. doi: 10.4061/2011/216464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Martinez V, Rivier J, Coy D, Tache Y. Intracisternal injection of somatostatin receptor 5-preferring agonists induces a vagal cholinergic stimulation of gastric emptying in rats. J Pharmacol Exp Ther. 2000;293:1099–105. [PubMed] [Google Scholar]
- 208.Helke CJ, Rabchevsky A. Axotomy alters putative neurotransmitters in visceral sensory neurons of the nodose and petrosal ganglia. Brain Res. 1991;551:44–51. doi: 10.1016/0006-8993(91)90911-e. [DOI] [PubMed] [Google Scholar]
- 209.Zhuo H, Sinclair C, Helke CJ. Plasticity of tyrosine hydroxylase and vasoactive intestinal peptide messenger RNAs in visceral afferent neurons of the nodose ganglion upon axotomy-induced deafferentation. Neuroscience. 1994;63:617–26. doi: 10.1016/0306-4522(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 210.Zhuo H, Lewin AC, Phillips ET, Sinclair CM, Helke CJ. Inhibition of axoplasmic transport in the rat vagus nerve alters the numbers of neuropeptide and tyrosine hydroxylase messenger RNA-containing and immunoreactive visceral afferent neurons of the nodose ganglion. Neuroscience. 1995;66:175–87. doi: 10.1016/0306-4522(94)00561-i. [DOI] [PubMed] [Google Scholar]
- 211.Saade NE, Abdallah LE, Barada KA, Atweh SF, Nassar CF. Effects of intracerebral injections of VIP on jejunal alanine absorption and gastric acid secretion in rats. Regul Pept. 1995;55:269–76. doi: 10.1016/0167-0115(94)00115-e. [DOI] [PubMed] [Google Scholar]
- 212.Sekizawa S, Joad JP, Bonham AC. Substance P presynaptically depresses the transmission of sensory input to bronchopulmonary neurons in the guinea pig nucleus tractus solitarii. J Physiol. 2003;552:547–59. doi: 10.1113/jphysiol.2003.051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Martin JL, Jenkins VK, Hsieh HY, Balkowiec A. Brain-derived neurotrophic factor in arterial baroreceptor pathways: implications for activity-dependent plasticity at baroafferent synapses. J Neurochem. 2009;108:450–64. doi: 10.1111/j.1471-4159.2008.05781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hsieh HY, Robertson CL, Vermehren-Schmaedick A, Balkowiec A. Nitric oxide regulates BDNF release from nodose ganglion neurons in a pattern-dependent and cGMP-independent manner. J Neurosci Res. 2010;88:1285–97. doi: 10.1002/jnr.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]