Abstract
AIM: To determine significant indicators for the efficacy of sorafenib in patients with advanced hepatocellular carcinoma (HCC).
METHODS: A total of 46 patients with Barcelona Clinic Liver Cancer stage C who received sorafenib for more than 30 d at the Iizuka Hospital from June 2009 to December 2012 were enrolled in this study. Multivariate and univariate analyses were performed to evaluate the associations of hepatic function according to Child-Pugh grade, location and size of the largest tumor and adverse events of sorafenib treatment, such as hand-foot syndrome (HFS), hypertension, diarrhea, and alopecia, with the efficacy of treatment, as measured by overall survival (OS) and time to progression (TTP).
RESULTS: Patients included 39 men and 7 women whose ages ranged from 48 to 85 years (70.6 ± 9.6 years). HCC was classified according to etiology as follows: hepatitis C virus (n = 26), hepatitis B virus (n = 9), and other (n = 11). Liver function in patients was categorized as Child-Pugh grade A (n = 30) or B (n = 16). Tumors were categorized by size [< 5 cm (n = 33) or >5 cm (n = 13)] and the location of the largest tumor was used to categorize patients with intrahepatic (n = 28) or extrahepatic (n = 18) HCC. HFS, hypertension, diarrhea, and alopecia were present in 22 (47.8%), 19 (41.3%), 15 (32.6%) and 7 patients (15.2%), respectively. The median OS of all patients was 373 d and the median TTP was 112 d. The etiology of HCC did not correlate with the median OS and TPP. The median OS of patients with tumors < 5 cm was significantly longer than those with larger tumors (496 vs 245 d; HR = 0.19, 95%CI: 0.07-0.48; P < 0.01). According to the results of a multivariate analysis, the size of the largest tumor affected OS (HR = 0.22, 95%CI: 0.08-0.59; P < 0.01). The median TTP was significantly longer in patients with extrahepatic compared to intrahepatic major HCC (224 vs 98 d; HR = 0.32; 95%CI: 0.14-0.67; P < 0.01). The median TTP of patients with HFS was significantly longer than those without it (195 d vs 83 d; HR = 0.41, 95%CI: 0.20-0.82; P < 0.05), and the median TTP was significantly longer in patients with hypertension (195 d vs 84 d; HR = 0.43, 95%CI: 0.21-0.84; P < 0.05). According to the results of the multivariate analysis, extrahepatic major HCC (HR = 0.36, P < 0.01) and HFS (HR = 0.44, P < 0.05) prolonged TTP.
CONCLUSION: Extrahepatic major HCC and HFS are associated with prolonged TTP and are useful indicators for judging the efficacy of sorafenib treatment.
Keywords: Hand-foot syndrome, Hepatocellular carcinoma, Indicator of efficacy, Sorafenib, Time to progression
Core tip: Clinical factors influencing the efficacy of sorafenib for treating patients with advanced hepatocellular carcinoma (HCC) were evaluated in this study. As only the size of the largest tumor correlated significantly with overall survival, analyses were focused on factors affecting time to progression (TTP). Sorafenib was more effective for treating patients with extrahepatic compared to intrahepatic advanced HCC. The occurrence of hand-foot syndrome as an adverse event significantly correlated with increased TTP and may therefore be a useful indicator for adjusting the dose of sorafenib to improve its effectiveness and the health-related quality of life of patients with advanced HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary liver cancer, developing in more than one million new patients each year worldwide, and is the fifth most common cancer and the third most common cause of cancer-related death[1]. The Barcelona Clinic Liver Cancer staging system (BCLC)[2] is widely used to assist in the selection of HCC treatment, which is determined by the tumor characteristics, such as size, number, and presence of vascular invasion or extrahepatic metastasis, as well as by the patient’s hepatic function and performance status. Curative treatments, including surgical resection and radiofrequency ablation, are considered for patients with early stage HCC (solitary tumor < 5 cm or as many as three nodules < 3 cm in diameter) and well-preserved hepatic function[3,4]. Advanced HCC can be treated with sorafenib, an orally administered inhibitor of multiple protein kinases, such as c-Raf, B-Raf, mitogen-activated protein kinase kinase, extracellular signal regulated kinase, and vascular endothelial growth factor[5,6]. Sorafenib induces apoptosis of tumor cells and inhibits tumor angiogenesis[7]. The phase III Sorafenib HCC Assessment Randomized Protocol (SHARP) trial demonstrated that median overall survival (OS) and time to progression (TTP) of patients with advanced HCC is improved with sorafenib compared with placebo[8]. Sorafenib is therefore the recommended first-line treatment for patients with BCLC stage C HCC[3,4].
Despite the success of sorafenib for the treatment of advanced HCC[9-11], significant predictive factors for its efficacy are not available. Sorafenib treatment induces adverse events such as diarrhea, hand-foot syndrome (HFS) and hypertension[8,12]. Moreover, early skin reactions correlate with tumor control[13]. Although some adverse events may predict efficacy or indicate a need for dose adjustment, there are no standard guidelines to follow[8-13]. The aim of the present study was to determine the factors that influence the efficacy of sorafenib according to the Child-Pugh tumor grade[14] at the beginning of treatment, the location and the size of the largest tumor, and common adverse events such as HFS, hypertension, diarrhea, and alopecia.
MATERIALS AND METHODS
Patients
Eighty patients with advanced HCC were treated with sorafenib at the Iizuka Hospital from June 2009 to December 2012. Of these, 46 patients with BCLC stage C HCC who were treated with sorafenib for more than 30 d were enrolled. Patients included 39 men and 7 women with an average age of 70.6 ± 9.6 years (range: 48-85 years). The Ethics Committee of Iizuka Hospital approved this study.
Treatment and evaluations
At the beginning of treatment, patients were administered 800 mg (11/46; 23.9%), 400 mg (25/46; 54.3%), or 200 mg (10/46; 21.8%) of sorafenib each day. Adverse events were evaluated according to the United States National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.0 (palmar-planter erythrodysesthesia syndrome was defined as HFS). The dose of sorafenib was then adjusted between 200-800 mg according to the degree of adverse events and each patient’s tolerance. When grade 3 adverse events occurred, treatment was temporarily discontinued and resumed after improvement to < grade 2. The efficacy of treatment was evaluated from results of dynamic computed tomography or magnetic resonance imaging scans performed at intervals of six weeks, and by OS and TTP according to the modified Response Evaluation Criteria In Solid Tumors (RECIST) criteria[15].
Statistical analysis
Statistical analyses were performed using JMP software (version 8.0.2; SAS Institute Inc., Cary, NC, United States). OS and TTP were estimated using Kaplan-Meier analysis of subcategories according to etiology of liver cirrhosis, Child-Pugh grade, size and location of the largest tumor, and adverse events. Cox proportional hazards models were used to estimate the hazard ratio (HR) and 95%CI for each group. Data are presented as median values. A P value < 0.05 was considered statistically significant.
RESULTS
Characteristics of patients and treatment outcomes
At the beginning of treatment, patient liver function was designated as Child-Pugh grade A (CP-A) (n = 30) or B (CP-B) (n = 16). The major sites of HCC were intrahepatic (n = 28) or extrahepatic (n = 18), and etiologies were described as from hepatitis C virus (n = 26), hepatitis B virus (n = 9), or other (n = 11). Patients were divided into two groups according to the diameter of the largest tumor: < 5 cm (Tmax < 5; n = 33) and > 5 cm (Tmax > 5; n = 13). The most frequent adverse event was HFS, which occurred in 22 patients (47.8%). Hypertension, diarrhea, and alopecia occurred in 19 (41.3%), 15 (32.6%), and 7 patients (25.2%), respectively (Table 1). Median OS and TTP were 373 and 112 d, respectively, for all patients (Figure 1).
Table 1.
Incidence of adverse events
| NTC-CTCAE grade1 (1/2/3/4/5) | |
| Hand-foot syndrome | 12/7/3/0/0 |
| Hypertension | 12/7/0/0/0 |
| Diarrhea | 10/5/0/0/0 |
| Alopecia | 7/0/-/-/- |
The highest grade of adverse events was described. NTC-CTCAE: National Cancer Institute’s Common Terminology Criteria for Adverse Events.
Figure 1.
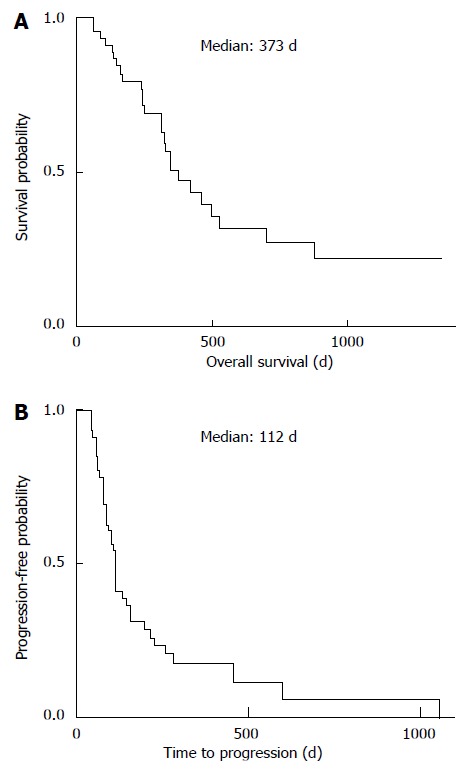
Overall survival and time to progression. A: Overall survival; B: Time to progression of all patients enrolled in this study was estimated using Kaplan-Meier analysis.
Factors contributing to OS
Results show that the median OS did not correlate with the etiology of HCC (Figure 2A). However, the median OS of CP-A patients was significantly longer than those with CP-B (462 d vs 242 d; P < 0.01) (Table 2). Furthermore, the median OS of patients with Tmax < 5 was significantly longer than those with Tmax > 5 (496 d vs 245 d; P < 0.01), and a multivariate analysis indicated that tumors > 5 cm significantly affected OS (P < 0.01) (Table 2).
Figure 2.
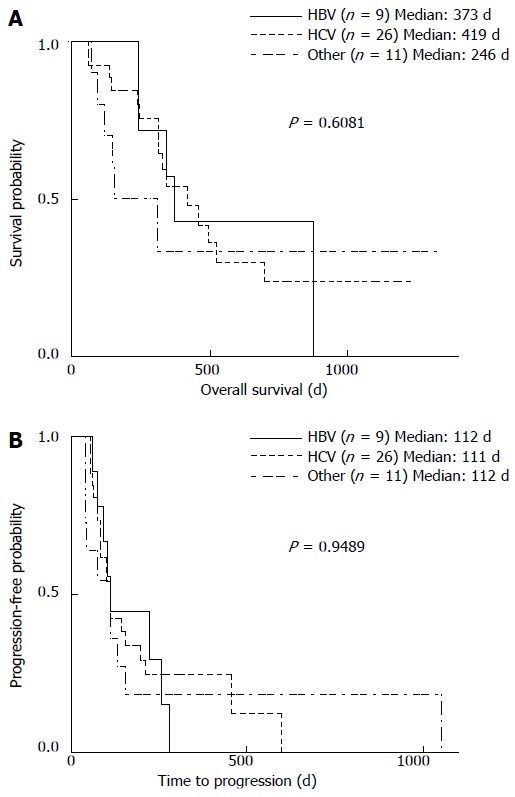
Hepatocellular carcinoma etiology in relation to overall survival and time to progression. A: Overall survival (OS); B: Time to progression (TTP) were analyzed according to the etiology of hepatocellular carcinoma (HCC). HBV: Hepatitis B virus; HCV: Hepatitis C virus.
Table 2.
Cox proportional hazards model analysis of overall survival
| Variable |
Univariate |
Multivariate |
||
| HR (95%CI) | P | HR (95%CI) | P | |
| Child-Pugh grade | ||||
| A | 0.26 (0.13-0.70) | 0.0062a | 0.42 (0.17-1.04) | 0.0594 |
| B | 1.00 | 1.00 | ||
| Major HCC | ||||
| Intrahepatic | 1.00 | 1.00 | ||
| Extrahepatic | 0.39 (0.13-0.97) | 0.0430a | 0.57 (0.18-1.52) | 0.2757 |
| Largest tumor | ||||
| < 5 cm | 0.19 (0.07-0.48) | 0.0007a | 0.22 (0.08-0.59) | 0.0030a |
| > 5 cm | 1.00 | 1.00 | ||
| Hand-foot syndrome | ||||
| - | 1.00 | 1.00 | ||
| + | 0.36 (0.15–0.80) | 0.0119a | 0.63 (0.24-1.62) | 0.3481 |
| Hypertension | ||||
| - | 1.00 | 1.00 | ||
| + | 0.40 (0.16-0.93) | 0.0321a | 0.69 (0.24-1.76) | 0.4441 |
| Diarrhea | ||||
| - | 1.00 | |||
| + | 0.61 (0.41-1.36) | 0.4644 | ||
| Alopecia | ||||
| - | 1.00 | |||
| + | 0.49 (0.13-1.36) | 0.1794 | ||
CI: Confidence interval; HCC: Hepatocellular carcinoma; HR: Hazard ratio.
P < 0.05 vs control.
Factors contributing to TTP
The etiology of HCC was not associated with the median TTP (Figure 2B). Median TTP also did not differ between patients with CP-A or CP-B grade liver function (113 d vs 104 d) (Figure 3A, Table 3). However, the median TTP of patients with extrahepatic major HCC was significantly longer compared with those with intrahepatic major HCC (224 d vs 98 d; P < 0.01) (Figure 3B), which was also significant by a multivariate analysis (P < 0.01) (Table 3). Tumor size was not associated with TTP, with no significant difference between patients with Tmax < 5 and Tmax > 5 (112 vs 98 d) (Figure 3C, Table 3).
Figure 3.
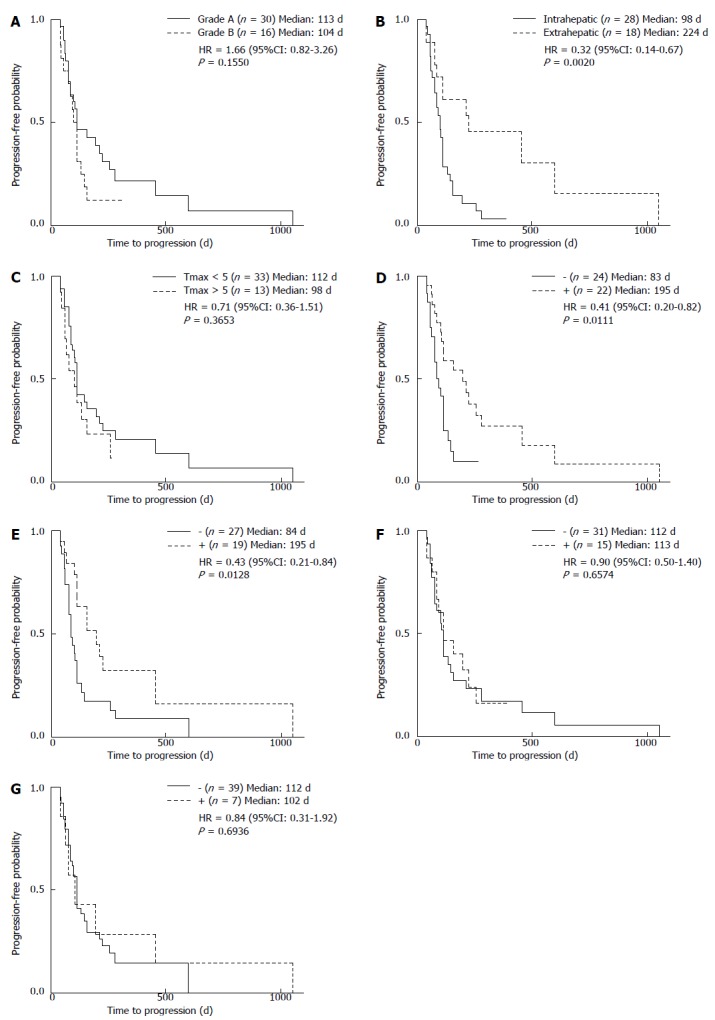
Relationship of clinical factors with time to progression. Time to progression (TTP) was analyzed according to A: Child-Pugh grade; B: Location of largest hepatocellular carcinoma; C: Size of the largest tumor, and the occurrence of D: Hand-foot syndrome; E: Hypertension; F: Diarrhea; G: Alopecia.
Table 3.
Cox proportional hazards model analysis of time to progression
| Variable |
Univariate |
Multivariate |
||
| HR (95%CI) | P | HR (95%CI) | P | |
| Child-Pugh grade | ||||
| A | 0.60 (0.31-1.22) | 0.1550 | ||
| B | 1.00 | |||
| Major HCC | ||||
| Intrahepatic | 1.00 | 1.00 | ||
| Extrahepatic | 0.32 (0.14-0.67) | 0.0020a | 0.36 (0.16-0.77) | 0.0070a |
| Largest tumor | ||||
| < 5 cm | 0.71 (0.36-1.52) | 0.3653 | ||
| > 5 cm | 1.00 | |||
| Hand-foot syndrome | ||||
| - | 1.00 | 1.00 | ||
| + | 0.41 (0.20-0.82) | 0.0111a | 0.44 (0.21-0.91) | 0.0274a |
| Hypertension | ||||
| - | 1.00 | 1.00 | ||
| + | 0.43 (0.21-0.84) | 0.0128a | 0.55 (0.26-1.09) | 0.0852 |
| Diarrhea | ||||
| - | 1.00 | |||
| + | 0.90 (0.54-1.40) | 0.6574 | ||
| Alopecia | ||||
| - | 1.00 | |||
| + | 0.84 (0.31-1.92) | 0.6936 | ||
CI: Confidence interval; HCC: Hepatocellular carcinoma; HR: Hazard ratio.
P < 0.05 vs control.
The median TTP of patients with HFS was significantly longer when compared with those without it (195 d vs 83 d; P < 0.05) (Figure 3D, Table 3). In addition, patients with hypertension had a significantly longer TTP compared to those without (195 d vs 84 d; P < 0.05) (Figure 3E, Table 3). However, median TTP did not differ in patients with or without diarrhea (113 d vs 112 d) (Figure 3F), or in patients with or without alopecia (102 d vs 112 d) (Figure 3G). Moreover, a univariate analysis showed that the median TTP did not differ significantly among patients receiving 200, 400, or 800 mg of sorafenib per day at the beginning of the treatment (95 d, 112 d, and 102 d, respectively) (Figure 4).
Figure 4.
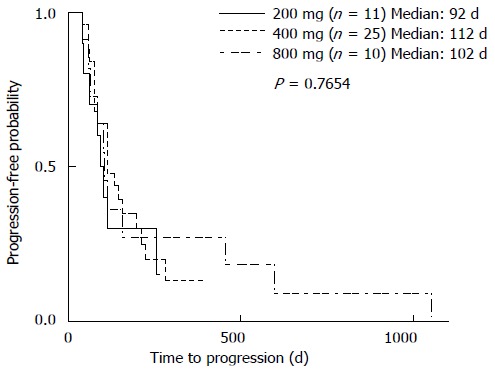
Relationship of starting sorafenib dose with time to progression. Time to progression (TTP) was analyzed according to sorafenib dose at the beginning of the treatment.
Comparison of clinical factors by location of major HCC
Clinical factors were compared between patients with intrahepatic and extrahepatic major HCC (Table 4). The median diameter of the largest tumor was significantly larger in patients with intrahepatic major HCC compared to those with extrahepatic major HCC (P < 0.01). Portal invasion of HCC was also more frequently observed in patients with intrahepatic major HCC (P < 0.01). However, patient age or sorafenib dose were not correlated with location of major HCC.
Table 4.
Comparison of clinical factors by location of major hepatocellular carcinoma
| Intrahepatic (n = 28) | Extrahepatic (n = 18) | P | |
| Age (yr) (mean ± SD) | 68.7 ± 8.5 | 73.6 ± 10.7 | 0.0500 |
| Largest tumor (cm) [median (range)] | 4.0 (1.5-15) | 1.0 (0.0-5.0) | < 0.0001a |
| Portal invasion (%) | 64.2 (18/28) | 5.7 (1/18) | < 0.0001a |
| Sorafenib | |||
| 800 mg | 7 | 4 | 0.9769 |
| 400 mg | 15 | 10 | |
| 200 mg | 6 | 4 |
SD: Standard deviation.
P < 0.05 vs control.
DISCUSSION
Results of the SHARP trial demonstrated that sorafenib was the first agent to improve the median OS and TTP of patients with advanced HCC[8]. The present study evaluated the impact of clinical factors on the efficacy of sorafenib for treating patients with BCLC stage C HCC. The median OS of patients was 373 d, which is comparable to the median OS of 10.5 mo reported in the SHARP trial. Multivariate analysis showed that the size of the largest tumor was the only factor that correlated significantly with OS, though CP-A was associated with a prolonged OS according to a univariate analysis. Hepatic function affects OS[16], consistent with untreated or nonsurgical HCC[17]. In contrast to OS, Child-Pugh grade was not associated with TTP, suggesting that hepatic function at the beginning of treatment did not determine the effect of sorafenib.
As previous reports indicated that the health-related quality of life (HRQoL) of patients with HCC gradually worsens because of the development of new symptoms as the disease progresses[18-20], this study focused on factors that might influence TTP. The results suggest that sorafenib is more effective in patients with extrahepatic major HCC than in those with intrahepatic major HCC. It is possible that different mechanisms of angiogenesis are involved in the progression of intrahepatic lesions and extrahepatic metastases. For example, many intrahepatic lesions are adequately supplied with blood by the hepatic arterial system, which primarily feeds liver tissue. However, vigorous de novo angiogenesis is required for the growth of metastatic tumors[21]. Thus, the progression of extrahepatic metastases likely requires greater angiogenic activity to provide sufficient blood flow, allowing for a more evident anti-angiogenic effect of sorafenib. Moreover, patients with intrahepatic major HCC had larger intrahepatic tumors and more frequent portal invasion than those with extrahepatic major HCC. As a result, hepatic arterial blood flow may have been increased, attenuating the efficacy of sorafenib.
Univariate analyses of the adverse events caused by sorafenib indicated that HFS and hypertension correlated significantly with TTP. Moreover, HFS was found to significantly affect TTP by a multivariate analysis. HFS is a frequent and typical form of dermatologic toxicity associated with multi-targeted kinase inhibitors (MKIs) such as sorafenib and sunitinib[22-24]. Although the precise mechanism by which MKIs cause HFS is unknown, the anti-angiogenic activity of MKIs may inhibit vascular repair mechanisms in high-pressure areas such as the palms and soles, which are repeatedly exposed to subclinical trauma[25]. These observations provide a possible rationale for concluding that HFS predicts a good response to the anti-angiogenic activity of sorafenib. Although the severe symptoms of HFS are not life-threatening, they diminish the HRQoL of patients receiving sorafenib[25,26]. As the results of this study show that the dose of sorafenib did not affect the median TTP, we recommend careful monitoring of the patient for signs of HFS, and adjusting the dose of sorafenib accordingly.
The progression of HCC causes symptoms of liver failure such as jaundice, refractory ascites, hepatic encephalopathy, and gastrointestinal bleeding, as well as intractable pain and cachexia. Therefore, extending the TTP can delay the appearance of symptoms of advanced HCC. This study shows that sorafenib was more effective in extending TTP in patients with extrahepatic major HCC. Moreover, HFS significantly correlated with increased TTP and is therefore a useful indicator for adjusting the dose of sorafenib. Sorafenib treatment should be individualized to improve its effectiveness and the HRQoL of patients with advanced HCC.
ACKNOWLEDGMENTS
We are grateful to Y. Ishibashi for assistance with manuscript preparation.
COMMENTS
Background
Although sorafenib is highly effective for treating patients with advanced hepatocellular carcinoma (HCC), significant predictive factors for its efficacy are not available.
Research frontiers
In the area of treatment for advanced hepatocellular carcinoma, the relationship of clinical factors with the efficacy of sorafenib is of great interest.
Innovations and breakthroughs
This study focused on hepatic function according to Child-Pugh grade, the location and the size of the largest tumor, and common adverse events such as hand-foot syndrome (HFS), hypertension, diarrhea, and alopecia, to evaluate the efficacy of sorafenib treatment of HCC. Sorafenib was found to be more effective in patients with extrahepatic major HCC compared to those with HCC in intrahepatic locations. Furthermore, HFS was found to significantly correlate with increased time to progression of HCC.
Applications
The results of this study suggest that HFS is a useful indicator for adjusting the dose of sorafenib.
Terminology
Sorafenib is an orally administered inhibitor of multiple protein kinases that induces apoptosis of tumor cells and inhibits tumor angiogenesis. The Phase III Sorafenib HCC Assessment Randomized Protocol trial showed that sorafenib improves the prognosis of patients with advanced HCC and is therefore recommended as a first-line treatment.
Peer review
In this paper, the authors examined the relationship between clinical factors and the sorafenib efficacy in HCC patients. The results of this study are of interest as they suggest a method for providing safer and more efficient sorafenib-based therapy.
Footnotes
P- Reviewer: Gherlan GS, Matsuda Y S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
References
- 1.Waly Raphael S, Yangde Z, Yuxiang C. Hepatocellular carcinoma: focus on different aspects of management. ISRN Oncol. 2012;2012:421673. doi: 10.5402/2012/421673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56 Suppl 1:S75–S87. doi: 10.1016/S0168-8278(12)60009-9. [DOI] [PubMed] [Google Scholar]
- 5.Strumberg D. Preclinical and clinical development of the oral multikinase inhibitor sorafenib in cancer treatment. Drugs Today (Barc) 2005;41:773–784. doi: 10.1358/dot.2005.41.12.937959. [DOI] [PubMed] [Google Scholar]
- 6.Adnane L, Trail PA, Taylor I, Wilhelm SM. Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 2006;407:597–612. doi: 10.1016/S0076-6879(05)07047-3. [DOI] [PubMed] [Google Scholar]
- 7.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 9.Wang SX, Byrnes A, Verma S, Pancoast JR, Rixe O. Complete remission of unresectable hepatocellular carcinoma treated with reduced dose of sorafenib: a case report. Target Oncol. 2010;5:59–63. doi: 10.1007/s11523-010-0133-x. [DOI] [PubMed] [Google Scholar]
- 10.Sacco R, Bargellini I, Gianluigi G, Bertini M, Bozzi E, Altomare E, Battaglia V, Romano A, Bertoni M, Capria A, et al. Complete response for advanced liver cancer during sorafenib therapy: case report. BMC Gastroenterol. 2011;11:4. doi: 10.1186/1471-230X-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inuzuka T, Nishikawa H, Sekikawa A, Takeda H, Henmi S, Sakamoto A, Saito S, Kita R, Kimura T, Osaki Y, et al. Complete response of advanced hepatocellular carcinoma with multiple lung metastases treated with sorafenib: a case report. Oncology. 2011;81 Suppl 1:152–157. doi: 10.1159/000333279. [DOI] [PubMed] [Google Scholar]
- 12.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 13.Vincenzi B, Santini D, Russo A, Addeo R, Giuliani F, Montella L, Rizzo S, Venditti O, Frezza AM, Caraglia M, et al. Early skin toxicity as a predictive factor for tumor control in hepatocellular carcinoma patients treated with sorafenib. Oncologist. 2010;15:85–92. doi: 10.1634/theoncologist.2009-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albers I, Hartmann H, Bircher J, Creutzfeldt W. Superiority of the Child-Pugh classification to quantitative liver function tests for assessing prognosis of liver cirrhosis. Scand J Gastroenterol. 1989;24:269–276. doi: 10.3109/00365528909093045. [DOI] [PubMed] [Google Scholar]
- 15.Edeline J, Boucher E, Rolland Y, Vauléon E, Pracht M, Perrin C, Le Roux C, Raoul JL. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer. 2012;118:147–156. doi: 10.1002/cncr.26255. [DOI] [PubMed] [Google Scholar]
- 16.Zugazagoitia J, Manzano A, Sastre J, Ladero JM, Puente J, Díaz-Rubio E. Sorafenib for non-selected patient population with advanced hepatocellular carcinoma: efficacy and safety data according to liver function. Clin Transl Oncol. 2013;15:146–153. doi: 10.1007/s12094-012-0902-3. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 18.Qiao CX, Zhai XF, Ling CQ, Lang QB, Dong HJ, Liu Q, Li MD. Health-related quality of life evaluated by tumor node metastasis staging system in patients with hepatocellular carcinoma. World J Gastroenterol. 2012;18:2689–2694. doi: 10.3748/wjg.v18.i21.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun V, Ferrell B, Juarez G, Wagman LD, Yen Y, Chung V. Symptom concerns and quality of life in hepatobiliary cancers. Oncol Nurs Forum. 2008;35:E45–E52. doi: 10.1188/08.ONF.E45-E52. [DOI] [PubMed] [Google Scholar]
- 20.Xie Y, Zhao FH, Lu SH, Huang H, Pan XF, Yang CX, Qiao YL. Assessment of quality of life for the patients with cervical cancer at different clinical stages. Chin J Cancer. 2013;32:275–282. doi: 10.5732/cjc.012.10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinstat-Saslow D, Steeg PS. Angiogenesis and colonization in the tumor metastatic process: basic and applied advances. FASEB J. 1994;8:401–407. doi: 10.1096/fasebj.8.6.7513289. [DOI] [PubMed] [Google Scholar]
- 22.Grandinetti CA, Goldspiel BR. Sorafenib and sunitinib: novel targeted therapies for renal cell cancer. Pharmacotherapy. 2007;27:1125–1144. doi: 10.1592/phco.27.8.1125. [DOI] [PubMed] [Google Scholar]
- 23.Chu D, Lacouture ME, Fillos T, Wu S. Risk of hand-foot skin reaction with sorafenib: a systematic review and meta-analysis. Acta Oncol. 2008;47:176–186. doi: 10.1080/02841860701765675. [DOI] [PubMed] [Google Scholar]
- 24.Chu D, Lacouture ME, Weiner E, Wu S. Risk of hand-foot skin reaction with the multitargeted kinase inhibitor sunitinib in patients with renal cell and non-renal cell carcinoma: a meta-analysis. Clin Genitourin Cancer. 2009;7:11–19. doi: 10.3816/CGC.2009.n.002. [DOI] [PubMed] [Google Scholar]
- 25.Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, Garbe C, Hauschild A, Puzanov I, Alexandrescu DT, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001–1011. doi: 10.1634/theoncologist.2008-0131. [DOI] [PubMed] [Google Scholar]
- 26.Sibaud V, Dalenc F, Chevreau C, Roché H, Delord JP, Mourey L, Lacaze JL, Rahhali N, Taïeb C. HFS-14, a specific quality of life scale developed for patients suffering from hand-foot syndrome. Oncologist. 2011;16:1469–1478. doi: 10.1634/theoncologist.2011-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]


