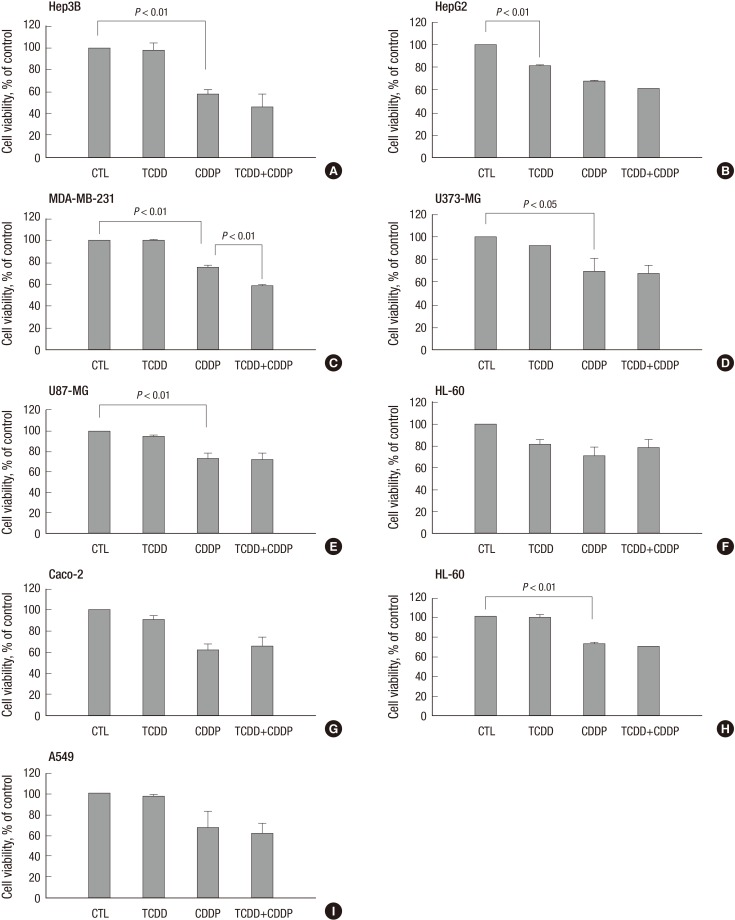
Fig. 2.
Decrease in cell viability of cisplatin-treated liver (Hep3B) and breast (MDA-MB-231) cancer cells pretreatment with TCDD. Cells were pretreated with TCDD for two days: (A) liver (Hep3B), (D, E) brain (U373-MG, U87-MG), (F) blood (HL-60) and (H, I) lung (H460, A549) cancer cells were treated with 10 nM TCDD; (B) liver (HepG2) and (C) breast (MDA-MB-231) cells were treated with 5 nM, and (G) colon (Caco-2) cells were treated with 4 nM TCDD. Subsequently one-day treatment with cisplatin was 5 µg/mL for Hep3B, MDA-MB-231 and A549 cells, 15 µg/mL for U373-MG, U87-MG, and HL-60 cells and 10 µg/mL for H460 cells. HepG2 and Caco-2 cells were treated with 4 and 40 µg/mL cisplatin, respectively. Control cells were treated as described for Fig. 1. Cell viability is expressed as the mean ± SD from three independent experiments performed in triplicate. Statistical significance (P values) is indicated for each Figure. CTL, control; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; CDDP, cisplatin.