Fig. 6.
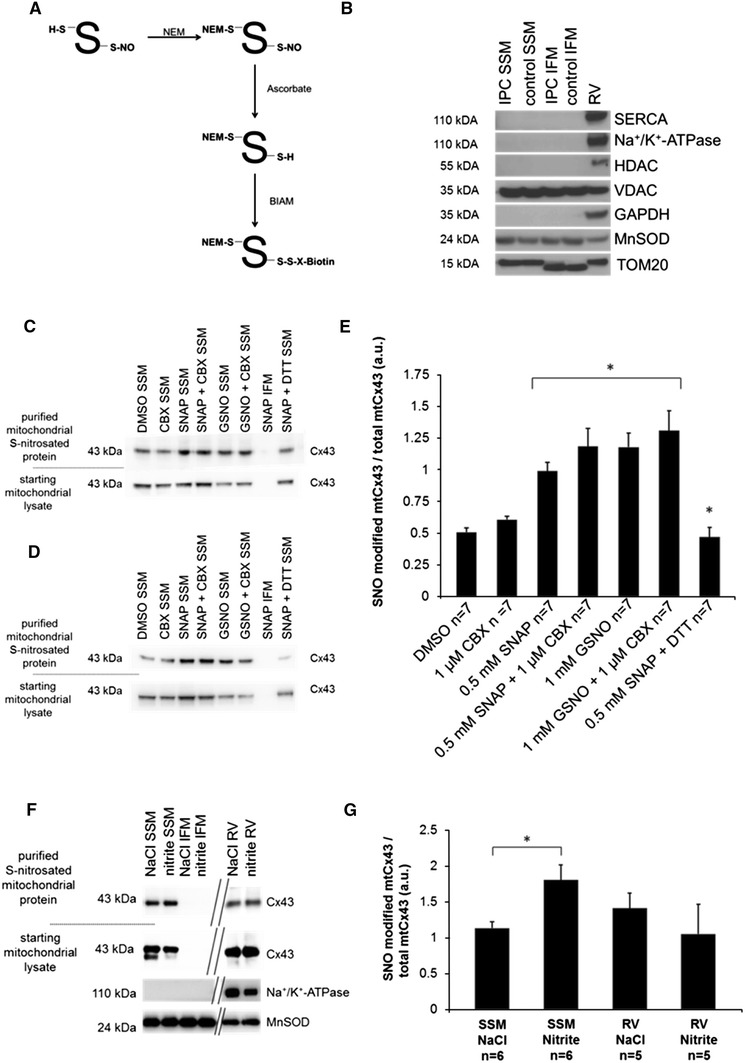
SNO quantification of mtCx43 for NO treated mitochondria. SNO quantification was performed on mitochondria treated either with carbenoxolone (CBX), S-nitroso-N-acetyl-dl-penicillamine (SNAP), S-nitrosoglutathione (GSNO), a combination of NO donor and hemichannel blocker (SNAP + CBX; GSNO + CBX), or dimethyl sulfoxide (DMSO; used as solvent). Using a modified biotin switch method, SNO residues of mitochondrial proteins were labeled with biotin (a). Following mitochondrial isolation by Percoll gradient ultracentrifugation, purity of SSM and IFM preparations was determined by the absence of immunoreactivity for antibodies directed against markers of the plasma membrane (Na+/K+-ATPase), sarcoplasmic reticulum (SERCA2 ATPase), nucleus (HDAC), cytosol (GAPDH), and enrichment of mitochondrial proteins (VDAC, MnSOD, and TOM20) (b). After precipitation of biotin labeled SNO-modified proteins, Western blot analysis was performed for mtCx43 (c). Biotin labeled mitochondria were treated with 10 mmol DTT (c) or 100 mmol DTT (d) and used as a negative control. Band signal intensity of purified SNO-modified mtCx43 was normalized to a loading control taken from the starting mitochondrial lysate representing the total amount of loaded mtCx43 (c, d). Additionally, SNO of mtCx43 was quantified subsequent to injection of sodium nitrite or an equal volume of NaCl into mouse left ventricles (f, g). Asterisk (p < 0.05) indicates significant difference to unmarked groups. Results are expressed as mean ± SEM of seven replicates per group
