Abstract
Legitimate use of legal intranasal decongestants containing l-methamphetamine may complicate interpretation of urine drug tests positive for amphetamines. Our study hypotheses were that commonly used immunoassays would produce no false-positive results and a recently developed enantiomer-specific gas chromatography–mass spectrometry (GC–MS) procedure would find no d-amphetamine or d-methamphetamine in urine following controlled Vicks VapoInhaler administration at manufacturer's recommended doses. To evaluate these hypotheses, 22 healthy adults were each administered one dose (two inhalations in each nostril) of a Vicks VapoInhaler every 2 h for 10 h on Day 1 (six doses), followed by a single dose on Day 2. Every urine specimen was collected as an individual void for 32 h after the first dose and assayed for d- and l-amphetamines specific isomers with a GC–MS method with >99% purity of R-(−)-α-methoxy-α-(trifluoromethyl)phenylacetyl derivatives and 10 µg/L lower limits of quantification. No d-methamphetamine or d-amphetamine was detected in any urine specimen by GC–MS. The median l-methamphetamine maximum concentration was 62.8 µg/L (range: 11.0–1,440). Only two subjects had detectable l-amphetamine, with maximum concentrations coinciding with l-methamphetamine peak levels, and always ≤4% of the parent's maximum. Three commercial immunoassays for amphetamines EMIT® II Plus, KIMS® II and DRI® had sensitivities, specificities and efficiencies of 100, 97.8, 97.8; 100, 99.6, 99.6 and 100, 100, 100%, respectively. The immunoassays had high efficiencies, but our first hypothesis was not affirmed. The EMIT® II Plus assay produced 2.2% false-positive results, requiring an enantiomer-specific confirmation.
Introduction
Amphetamines are an important component of many workplaces, judicial and clinical drug-testing programs because of their high abuse potential. For methamphetamine and amphetamine, stereoisomer determination is important for proper result interpretation. The S(+) or d-stereoisomers are strong central nervous system stimulants releasing dopamine from storage vesicles and interfering with dopamine transporter function. Methamphetamine and amphetamine have high abuse liability due to increased dopamine release in the extracellular synapse. The R(−) or l-isomers have milder dopaminergic effects, with l-methamphetamine marketed as a nasal decongestant in Vicks VapoInhaler. Failure to identify the correct methamphetamine stereoisomer in urine can result in incorrect interpretation of urine drug tests. An older case report described a patient accused of abusing an illicit drug until a specific gas chromatography–mass spectrometry (GC–MS) test found l-methamphetamine in his urine, confirming his assertion that he used Vicks VapoInhaler (1).
Most urine drug-testing programs in the 1980s utilized GC–MS for confirmation but did not have specific isomeric methods. Fitzgerald et al. (2) published one of the first GC–MS methods for distinguishing methamphetamine isomers in urine with N-trifluoroacetyl-l-prolyl (l-TPC) derivatives, and this remains the commonest method in US drug-testing programs today. One limitation of this assay is that the derivatizing reagent contained a small and variable amount of N-trifluoroacetyl-d-prolyl (d-TPC). d-TPC produced a derivative of l-methamphetamine that co-eluted with the l-TPC derivative of d-methamphetamine. This co-eluting compound could be mistaken for d-methamphetamine and was one reason that federal guidance to medical review officers required that a minimum of 20% d-methamphetamine was necessary to define a positive d-methamphetamine specimen (3). The manufacturer claims that Vicks VapoInhaler contains <1% d-methamphetamine; however, laboratories participating in National Laboratory Certification Program (NLCP) proficiency testing reported up to 2.5% d-methamphetamine in a Vicks VapoInhaler extract (Personal communication from Dr Francis Esposito, RTI International, Research Triangle, NC, NLCP Proficiency Program, April 2014). Paul et al. (4) published a method with R-(−)-α-methoxy-α-(trifluoromethyl)phenylacetyl (MTPA) chloride that was >99% pure for derivatization of amphetamines and methylenedioxyamphetamines. This method made possible the reexamination of Vicks VapoInhaler purity.
Peak l-methamphetamine urine concentrations from three subjects following Vicks VapoInhaler intake every 20 min for 6 h were 1,520, 1,950 and 6,000 µg/L (2). These doses were much higher than recommended, but demonstrated that individuals might have a false-positive methamphetamine urinalysis following Vicks VapoInhaler intake. A comprehensive study of the clinical pharmacology of intranasal l-methamphetamine in 12 human participants was conducted, including urine and blood concentrations (5). Two inhalations per nostril for four dosing sessions separated by 2 h each or 16 inhalations were administered according to manufacturer's instructions. Subsequent studies increased the dose to 32 and then 64 inhalations over 8 h. The primary purpose of urine measurements was to determine the total amount of drug excreted; therefore, each urine void was not collected. Urine was pooled from 0 to 12, 12 to 24 and 24 to 36 h. The mean mass of l-methamphetamine excreted for each 12 h period was ∼15, 25 and 50 µg for the 16, 32 and 64 inhalation conditions, respectively. These amounts represented total excretion of about half the administered dose as l-methamphetamine and 4% as l-amphetamine.
With the advent of alternative matrices to detect use or abuse of amphetamines, it became important to have a more detailed pharmacokinetic urine profile following controlled administration of Vicks VapoInhaler. It also was important to determine the frequency of false-positive results from commonly employed immunoassays. In many drug-testing programs, positive immunoassay results are confirmed with a GC–MS method that is not stereospecific. The percent of d- and l-methamphetamine is determined only if requested by the medical review officer. This study examined d- and l-methamphetamine and d- and l-amphetamine concentrations in urine specimens collected from the subjects inhaling Vicks VapoInhaler following manufacturer's recommended doses. Concentrations of S(+)/d- and R(−)/l-isomers of methamphetamine and amphetamine were quantified in each urine specimen by GC–MS. Each urine specimen also was analyzed by three commercial immunoassays for amphetamines that are commonly employed in federally mandated drug-testing programs.
Materials and methods
Participants
Subjects provided written informed consent to participate in this National Institute on Drug Abuse Intramural Research Program Institutional Review Board-approved study. Individuals were recruited by television, radio and newspaper advertisements, flyers and participant referrals. Participants received a comprehensive medical and psychological evaluation to verify compliance with eligibility criteria. Participants were 18–65 years of age with adequate peripheral venous access. Exclusion criteria included any current medical condition precluding safe study participation, current dependence on any psychoactive substance other than nicotine or caffeine or inability to tolerate intranasal administration.
Study design
Participants entered the secure research unit ≥2 h before dosing. A urine specimen was collected in a polypropylene container prior to dosing. An aliquot was analyzed for amphetamines with an iScreen (Blue Grass Drug Screen, Inc.), and a urine pregnancy test was performed for females. Subjects with positive results were excluded. In accordance with the manufacturer's recommended dosage, each participant on the first day inhaled from a Vicks VapoInhaler twice in each nostril every 2 h between 09:00 h and 19:00 h (total of 24 inhalations), and at 06:00 h on the second day (four inhalations). The inhaler contained 50 mg l-methamphetamine, with 0.04–0.15 mg administered per inhalation (total of up to 0.60 mg l-methamphetamine per dose). Every urine sample was collected ad libitum for 32 h after the first and 11 h after the last dose. The volume of each urine void was measured, 1 mL aliquoted for immunoassay testing and the remainder stored at −20°C for GC–MS testing. Urine analyses were performed by the United States Army Forensic Toxicology Drug Testing Laboratory, Fort Meade, MD 20755, USA, an NLCP-certified laboratory. Samples were analyzed blind by the Army laboratory and blind quality control samples, prepared by the Chemistry and Drug Metabolism Section, Intramural Research Program (IRP), NIDA, Baltimore, MD 21224, USA, were included within each batch. Blind quality control samples containing d- and l-isomers of methamphetamine and amphetamine prepared in urine at concentrations of 100 and 500 µg/L were distributed randomly among specimens from participants and analyzed along with the specimens.
Immunoassays
Specimens were thawed, transferred to barcode-labeled screening vials and analyzed on a Hitachi P or D Module Immunoanalyzer (Roche Diagnostics, Indianapolis, IN, USA). Three immunoassays were performed on each specimen: EMIT® II Plus Amphetamines Assay (Siemens AG, Erlangen, Germany), KIMS® Amphetamines II (Roche Diagnostics) and DRI® Amphetamines Assay (Microgenics Corporation, Fremont, CA, USA). The EMIT® II Plus Amphetamines manufacturer reports 38% cross-reactivity with l-methamphetamine and 13% with l-amphetamine. For KIMS® Amphetamines II, the manufacturer reported cross-reactivities of 11 and 4%, respectively. The manufacturer of DRI® Amphetamines Assay did not report cross-reactivities for the current cutoff concentration, but their package insert lists cross-reactivities for l-isomers as <10% at a cutoff of 1,000 µg/L. For each immunoassay method, the procedures followed manufacturers' recommended instructions with a cutoff concentration of 500 µg/L d-methamphetamine. The methods were validated in accordance with NLCP requirements (6). Quality control samples in each batch contained d-methamphetamine at 0, 75 and 125% cutoff concentrations.
Gas chromatography–mass spectrometry
All specimens regardless of immunoassay results were tested by GC–MS for l-amphetamine, d-amphetamine, l-methamphetamine and d-methamphetamine. Specimens were thawed and precise aliquots transferred to barcode-labeled tubes for GC–MS analyses. When quantifications were above the upper limit of linearity (ULOL), specimens were re-aliquoted, diluted and analyzed to obtain results based on data in the linear range.
The analytical method, a previously published GC–MS SIM procedure using MTPA derivatization (4) with minor modifications, is briefly described. The method employed a single calibrator with 40 µg/L of each isomer of amphetamine and methamphetamine and d11 racemic amphetamine and d14 racemic methamphetamine (40 µg/L each isomer) internal standards. Further, 750 µL 1 M phosphate buffer (pH 9) and 750 µL 0.4 M periodate solution were added to 2 mL urine, incubated for 15 min at 60°C and extracted with Cerex Polycrom Clin II solid-phase extraction columns (SPEware). Analytes were eluted with 2 mL methylene chloride–acetone–triethylamine (80 : 20 : 2), and 20 µL R-(−)MTPA in acetonitrile (1 : 20) was added, heated for 15 min at 65°C, evaporated and reconstituted in 75 µL ethyl acetate. GC–MS SIM ions were (quantification ion in bold) amphetamine internal standard ions 264, 98; amphetamine 260, 162, 118; methamphetamine internal standards 281, 98 and methamphetamine 274, 200, 176. The method was revalidated in accordance with NLCP requirements (6). For each isomer, the lower limit of quantification (LLOQ), ULOL, within run imprecision and between run imprecision were 10, 750 µg/L, 1.9–3.1% and 3.3–5.1%, respectively. There was a concentration-dependent positive bias that was <11% at the ULOL for each analyte. There was no interference with the 16 µg/L l-methamphetamine or d-methamphetamine control sample from 1 g/L of phenylpropanolamine, ephedrine or pseudoephedrine, 50,000 µg/L phentermine or 5,000 µg/L of 3,4-methylenedioxyamphetamine, 3,4-methylenedioxymethamphetamine or 3,4-methylenedioxyethylamphetamine. Furthermore, 50,000 µg/L phentermine interfered with the amphetamine internal standard, precluding quantification for either isomer.
Creatinine
Creatinine concentrations were measured on a Hitachi P or D Module Analyzer (Roche Diagnostics) by the modified Jaffe method (Sciteck; Arden, NC, USA).
Data analysis
Immunoassay results were classified as positive if responses were ≥500 µg/L d-methamphetamine. A result was considered a false positive if the immunoassay was positive and the GC–MS confirmation result was <250 µg/L d-methamphetamine or d-amphetamine. A true positive had a positive immunoassay and a positive GC–MS result, a true-negative result was negative in both assays and a false negative had a negative immunoassay and positive GC–MS result. Sensitivity was defined as 100 times the number of true-positive results divided by the sum of true-positive and false-negative results (expressed as %). Specificity is 100 times the number of true-negative results divided by the number of true-negative and false-positive results. The efficiency of an assay was 100 times the sum of true-positive and true-negative results divided by the total number of results. To remove variability due to dilution, some pharmacokinetic parameters were determined from normalized data computed by dividing the drug concentration by the creatinine concentration and reporting as microgram drug per gram creatinine.
Results and discussion
Twenty-two subjects (15 men and 7 women; 5 white, 13 Black, 1 American Indian/Alaska Native and 3 more than one race) enrolled, of whom 17 completed the study. One subject missed the last dose, one missed the last two doses, one missed the first and third doses and one missed the fifth dose on Day 1. One subject vomited during the study and also was considered a non-completer. A total of 391 urine specimens were collected.
No initial specimen was methamphetamine or amphetamine positive. No d-isomers of methamphetamine or amphetamine were detected in any specimen by GC–MS. A total of 214 specimens (54.7%) had l-methamphetamine above the LLOQ with 25 (6.4%) ≥250 µg/L, the methamphetamine confirmation cutoff concentration in the federal drug-testing program. For the 17 subjects completing the study (n = 315 urine specimens), the median peak concentration (range) was 62.8 (11.0–1,440) µg/L (Table I). The median total l-methamphetamine excreted over the 32 h study from the 28 inhalations was 68.1 µg, which was similar to that reported by Mendelson et al. (5) of 68.6 µg after 32 inhalations. The median time to peak concentration was 10.6 h, near the time of the sixth and final dose on Day 1. The concentrations for this determination were normalized to reduce the effect of urine dilution. Without normalization, most specimens had a peak l-methamphetamine concentration in the morning of Day 2, when urine was most concentrated.
Table I.
l-Methamphetamine Pharmacokinetic Parameters for 17 Participants Who Received 24 Inhalations on Days 1 and 4 on the Morning of Day 2 with All Urine Voids Collected Over the 32 h Study Period (n = 315)
| l-Methamphetamine | |
|---|---|
| Median (range) peak concentration (µg/L) | 62.8 (11.0–1,440) |
| Median (range) total excreted (µg) | 68.1 (5.5–3,321) |
| Median time to normalized peak (h) | 10.6 |
| Range time to first result >LLOQ (h) | 1.2–12 |
| Number participants any specimen >250 µg/L | 2 |
| Number participants >LLOQ at 11 h post-dosing | 11 |
Only two subjects had detectable l-amphetamine with maximum concentrations of 62.3 µg/L (4.3% of the l-methamphetamine concentration in the same specimen) and 22.8 µg/L (2.3% of the l-methamphetamine concentration). The total amount of l-amphetamine excreted for these two subjects was 3 and <1% of the amount of l-methamphetamine excreted, respectively.
Each participant had at least one specimen with l-methamphetamine above the LLOQ. Two participants produced the first detectable result after the first dose and two required all six Day 1 doses before producing a positive specimen. Ten of the 17 completers had a peak concentration in the specimen collected early on the second day just before or after the Day 2 dose administration. As mentioned, this result was primarily due to these specimens being more concentrated and when results were normalized using a creatinine correction, most peak concentrations occurred on the first day. Of 17 participants, 11 had detectable l-methamphetamine 11 h after the last dose. Only two participants produced concentrations of l-methamphetamine >250 µg/L. Specimens in this concentration range would impact drug-testing laboratories that use a dual confirmation procedure for identifying methamphetamine-positive urine specimens. The first method does not distinguish stereoisomers, and the results for methamphetamine concentrations of ≥250 µg/L are reported as positive to medical review officers. The medical review officers would need to request a separate isomer analysis to identify the drug as l-methamphetamine.
Twelve blind quality control samples containing 100 µg/L and 12 containing 500 µg/L of l-amphetamine, l-methamphetamine, d-amphetamine and d-methamphetamine were analyzed. All analytes were correctly identified, with GC–MS concentrations for l-methamphetamine within ±20% of the target. l-Amphetamine, d-amphetamine and d-methamphetamine concentrations were within +7 to +28% of expected values.
Each of the amphetamines immunoassays evaluated had efficiencies >97% (Table II). Blind quality control samples prepared in urine were analyzed along with participant specimens and included in this evaluation. Twelve of the blind quality control samples provided true-positive d-methamphetamine and d-amphetamine samples to the study set. The total number of specimens evaluated was 415, except for the KIMS amphetamines assay. This assay was removed from the market by Roche Diagnostics during the study, yielding 262 total samples. The three commercial immunoassays for amphetamines EMIT® II Plus, KIMS® II and DRI® had sensitivities, specificities and efficiencies of 100, 97.8, 97.8; 100, 99.6, 99.6 and 100, 100, 100%, respectively. Despite the high efficiency of each immunoassay, the EMIT® II Plus Amphetamines assay produced more false-positive results than anticipated based on the manufacturer's stated cross-reactivity. For example, two of the nine false-positive results had l-methamphetamine concentrations of 534 and 587 µg/L with l-amphetamine <24 µg/L and no d-isomers. Laboratories employing this technique might require more stereospecific confirmation analyses.
Table II.
Performance Characteristics of Amphetamines Immunoassays with a 500 µg/L Screening Cutoff and a GC–MS d-Methamphetamine and d-Amphetamine Confirmation Cutoff of 250 µg/L in Urine Samples Collected from Subjects after Vicks VapoInhaler Use
| Method | EMIT® II+ | KIMS® II | DRI® |
|---|---|---|---|
| N | 415 | 262a | 415 |
| True negative | 394 | 253 | 403 |
| True positive | 12 | 8 | 12 |
| False negative | 0 | 0 | 0 |
| False positive | 9 | 1 | 0 |
| Sensitivity (%) | 100 | 100 | 100 |
| Specificity (%) | 97.8 | 99.6 | 100 |
| Efficiency (%) | 97.8 | 99.6 | 100 |
aAssay removed from the market during the study.
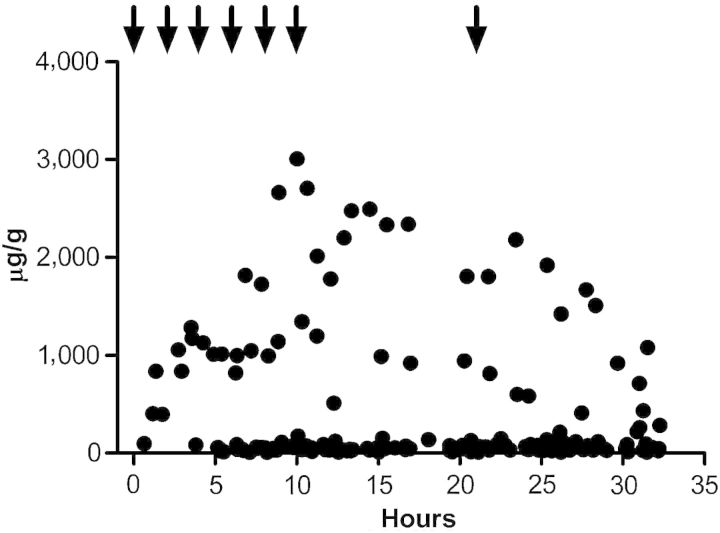
There were no false-positive drug tests for d-methamphetamine or d-amphetamine following Vicks VapoInhaler inhalations when testing was performed with the specific GC–MS procedure. There were substantial l-methamphetamine urine concentrations and fewer l-amphetamine-positive urine tests after dosing according to manufacturer's instructions. Vicks VapoInhaler appears to contain no d-methamphetamine or an amount too small to be detected in human urine following multiple inhalations. l-Methamphetamine was present in the product and in urine samples, concentrations were <1,500 µg/L. l-Methamphetamine was measureable in most urine specimens 11 h after the last inhalation but in a concentration of <250 µg/L. Large intersubject variability precluded determination of a mean elimination half-life. Figure 1 displays creatinine-normalized l-methamphetamine concentrations over the duration of the study. Even though concentrations reached 3,000 µg/g (1,440 µg/L), 70% of specimens were <300 µg/g or 93% <250 µg/L. Most of the specimens with concentrations of ≥250 µg/L were from one participant. There was no consistent trend in the concentrations following the last dose (Figure 1), at least within the 11-h timeframe of the study.
Figure 1.
Creatinine-normalized l-methamphetamine concentrations versus time after the first Vicks VapoInhaler inhalation for 17 subjects. Arrows indicate the time of each dose, i.e., two inhalations in each nostril/dose, with six doses separated by 2 h on Day 1 according to manufacturer's instructions and a single dose on Day 2.
Conclusion
After 28 Vicks VapoInhaler inhalations in accordance with the manufacturer's instructions, no d-methamphetamine or d-amphetamine was detected in urine at an LLOQ of 10 µg/L. l-Methamphetamine concentrations were as high as 1,440 µg/L, but the median peak concentration was 62.8 µg/L. Only 2 of 22 subjects had detectable l-amphetamine, with maximum concentrations coinciding with maximum l-methamphetamine concentrations, and ≤4% of the parent drug concentration. Only two participants had l-methamphetamine concentrations of ≥250 µg/L, the total methamphetamine cutoff concentration for federally regulated drug-testing programs. Three commercial immunoassays for amphetamines EMIT® II Plus, KIMS® II and DRI® had efficiencies of >97%. The high efficiencies of the immunoassays indicate that drug-testing laboratories screening with these methods will have few specimens requiring confirmation due to donor's inhalation of Vicks VapoInhaler. However, the EMIT® II Plus assay had more false-positive results than expected based on the manufacturer's reported cross-reactivity.
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Drug Abuse and the Division of Workplace Programs, Substance Abuse Mental Health Services Administration.
Acknowledgments
The authors thank Megan Taylor, Clinical Protocol Coordinator, CDM, IRP, NIDA, for her valuable assistance. The opinions in this article are those of the authors and do not necessarily reflect the views of the Department of Army or Department of Defense.
References
- 1.Solomon M.D., Wright J.A. False-positive for (+)-methamphetamine. Clinical Chemistry. 1977;23:1504. [PubMed] [Google Scholar]
- 2.Fitzgerald R.L., Ramos J.M., Jr., Bogema S.C., Poklis A. Resolution of methamphetamine stereoisomers in urine drug testing: urinary excretion of R(−)-methamphetamine following use of nasal inhalers. Journal of Analytical Toxicology. 1988;12:255–259. doi: 10.1093/jat/12.5.255. [DOI] [PubMed] [Google Scholar]
- 3.Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Substance Abuse Prevention. Medical review officer manual for federal agency workplace drug testing programs. 2010. October 1, p 52.
- 4.Paul B.D., Jemionek J., Lesser D., Jacobs A., Searles D.A. Enantiomeric separation and quantitation of (+/−)-amphetamine, (+/−)-methamphetamine, (+/−)-MDA, (+/−)-MDMA, and (+/−)-MDEA in urine specimens by GC-EI-MS after derivatization with (R)-(−) or (S)-(+)-alpha-methoxy-alpha-(trifluoromethyl)phenylacetyl chloride (MTPA) Journal of Analytical Toxicology. 2004;28:449–455. doi: 10.1093/jat/28.6.449. [DOI] [PubMed] [Google Scholar]
- 5.Mendelson J.E., McGlothlin D., Harris D.S., Foster E., Everhart T., Jacob P., III, et al. The clinical pharmacology of intranasal l-methamphetamine. BMC Clinical Pharmacology. 2008;8:4:1–9. doi: 10.1186/1472-6904-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Department of Health and Human Services. Mandatory guidelines for federal workplace drug testing programs. Federal Register. 2008;73:71858–71907. [Google Scholar]