Figure 9.
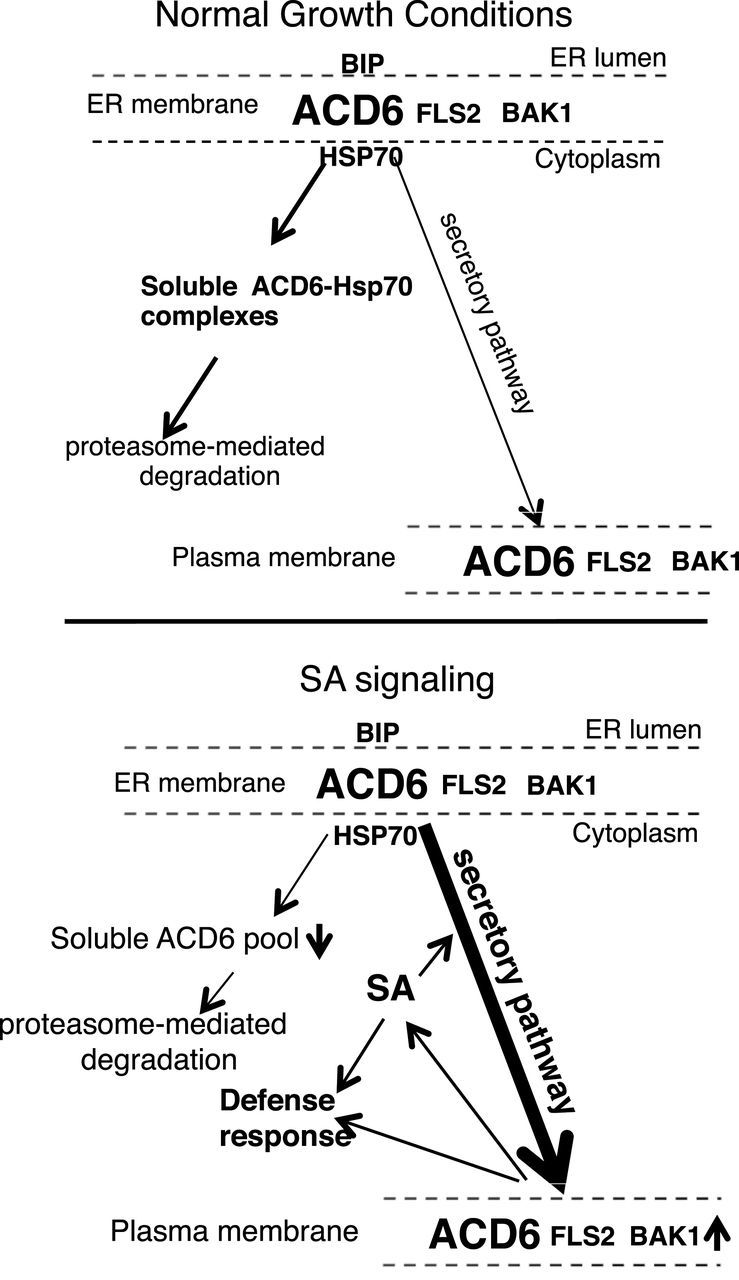
Working Model for the Regulation by SA of the Maturation and Localization/Transport of the ACD6 Protein and ACD6’s Co-Trafficking with FLS2 and BAK1.
Under normal growth conditions, plants have a low level of SA and ACD6 resides in large complexes (top panel). Maturation of ACD6 proteins and/or formation of productive complexes are not efficient and, as a consequence, a significant pool of ACD6 does not reach the plasma membrane. Instead, misfolded ACD6 and/or misassembled ACD6 complexes are constitutively retained in the ER and retrotranslocated into the cytosol (contained in the soluble fraction), where they form complexes with HSP70 and are degraded by the proteasome at a certain rate. This prevents the untimely activation of ACD6. Upon infection by some pathogens, plants accumulate a relatively high level of SA (lower panel), which regulates the efficiency of the maturation and transport of ACD6 proteins. As a result, the levels and sizes of ACD6 complexes and the amount of ACD6 transport to the plasma membrane are increased. SA signaling is also correlated with a reduction in the soluble pool of ACD6, which may be an indirect consequence of the increased efficiency of maturation. The increased plasma membrane pool of ACD6 ensures that cells with elevated SA can activate stronger defenses when exposed to a pathogen. FLS2 forms complexes with ACD6/ACD6-1. Additionally, FLS2 and its co-receptor BAK1 show increased abundance at the plasma membrane in response to SA, a process that depends strongly (for FLS2) or moderately (for BAK1) on ACD6. The model of regulation of ACD6 signaling by SA allows plants to finely tune its defense response to the invaders with minimal impact on plant growth.
