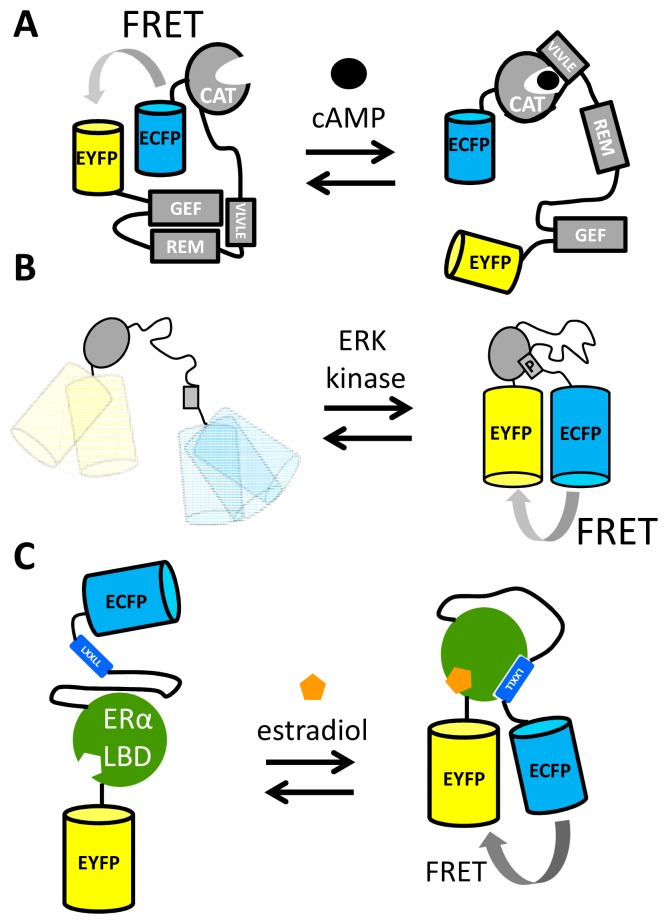
Figure 4.
Ligand-dependent protein-protein interactions of recognition domains often yield good FRET sensors. (A) A FRET sensor for cAMP exploiting the multidomain Epac1 cAMP-binding protein. The large conformational rearrangement of Epac1 upon binding cAMP results in a large change in FRET between fluorescent proteins fused to the termini of Epac1 [65]. (B) In kinase sensors, interaction between a phosphobinding domain and a phosphorylated substrate peptide typically results in an increase in FRET [74]. (C) A FRET sensor for agonists of the estrogen receptor uses the ligand binding domain of ERα, together with a co-activator peptide, to bring about a large increase in FRET between ECFP and EYFP upon an estradiol-binding induced co-activator-LBD interaction [76].