Abstract
Purpose
Data regarding endovascular treatment of chronic mesenteric ischemia (CMI) are sparse. Angioplasty is often accompanied by early restenosis and the need of further interventions. Thus we present our own patients’ data and review the recent literature.
Methods
We retrospectively reviewed all endovascular CMI treatments performed from 2008 to 2012 (27 patients, 14 females, mean age 70 ± 9 years). Patients’ data were retrieved from electronic health records. Patients’ follow-up included routinely performed color-coded ultrasonography, and, if performed for other clinical reasons, computed tomography or angiography. In addition, data extracted from 11 studies focusing on endovascular CMI treatment were analyzed.
Results
Procedures were performed without clinical complications in all patients. Seven patients received pure angioplasty and 20 patients stent-assisted angioplasties using bare metal stents, respectively. Three patients died 3, 5 and 32 months after the intervention. Five patients underwent re-intervention (one early restenosis at day 4 after pure angioplasty with stent placement and four because of in-stent restenosis, 5 to 23 months after placement). Another patient was treated surgically because of stent occlusion and reoccurring abdominal angina 15 months after the intervention. The 11 studies found in the literature included 429 cases with 196 treatments of the coeliac trunk (truncus coeliacus = TC), 319 of the superior mesenteric artery (SMA) and 42 of the inferior mesenteric artery (IMA). Patency rates in the more recent studies were high with up to 80% within 1 year. Data of earlier studies report longer follow-up periods and indicate low patency rates after three years. Our 2-year patency rate of 50% is within the range of reported patency data.
Conclusions
The presented data show that endovascular SMA treatment is a suitable and safe procedure in patients suffering from CMI, but long-term results are limited.
Keywords: chronic mesenteric ischemia, coeliac trunk, stent, superior mesenteric artery
Introduction
Chronic mesenteric ischemia (CMI) due to stenosis or occlusion of the superior mesenteric artery (SMA) is a rare disease. Ensuring sufficient blood supply from the common coeliac trunk (truncus coeliacus = TC) or the inferior mesenteric artery (IMA) can prevent symptomatic abdominal angina. In the past, abdominal bypass surgery was the primary treatment option for CMI; however, many of these patients have a high risk due to poor general state of health or severe concomitant disease. Thus bypass surgery is associated with a high perioperative morbidity and mortality and, therefore, is often unfeasible [1, 2]. The technical development of percutaneous approaches to treat atherosclerotic vessel disease has led to a first angioplasty of visceral arteries by Furrer et al. [3] in 1980. Ever since then the number of visceral interventions have increased worldwide.
The three visceral arteries affected by atherosclerotic lesions are TC, superior and inferior MA (SMA and IMA) with decreasing order of frequency [4]. SMA is treated the most frequently, because it is the most relevant artery associated with CMI and it is appropriate for angioplasty due to its anatomy [5]. If the SMA itself cannot be treated, TC can be chosen as a second target to increase collateral perfusion whereas IMA is avoided by most authors.
Nonetheless, due to limited number of cases the evidence for the decision to recommend interventional or open surgery of CMI is sparse. Visceral angioplasty is often complicated by early restenosis and the need of further interventions. Two reviews were published recently. Despite the same data basis the conclusions are quite different. Gupta et al. [6] concluded that “open revascularization surpasses endovascular procedures in long-term vessel patency and control of symptoms. The preferred revascularization approach used in treating this condition should be tailored to the anatomy and physiology of each patient.” Pecoraro et al. [7] concluded that “considering the lower periprocedural mortality and morbidity after endovascular treatment, this approach should be considered as the first treatment option in most CMI patients, especially in those with severe malnutrition. Primary open surgical treatment should be restricted to cases that do not qualify for endovascular treatment or good surgical risk patients with long life expectancy.”
As the number of endovascular treatments is still limited in these reviews (n = 714 [6], n = 1007 [7]), we present our own results after endovascular treatment in presence of CMI and give an overview of the recent literature about interventional treatments.
Methods
We retrospectively reviewed all cases of endovascular treatment of CMI performed in the five-year period from 2008 to 2012 at our hospital, a 1000-bed hospital in a rural area of Germany.
Twenty-seven patients (14 females, mean age 70 ± 9 years) were identified. Between 2008 and 2012, the number of interventions increased from 1 to 14. Patients’ data were extracted from their electronic health records. Details of interventions were reanalyzed from the digital data base. Patients’ follow-up included routinely performed color-coded ultrasonography and, if performed for other clinical reasons, computed tomography or angiography. Those who were not seen before were invited for a check-up that included the patient’s history, physical examination and duplex ultrasonography of visceral vessels. As a result, a recent imaging was available for almost every patient.
For literature reviews we performed a systematic search in PubMed database using the keywords “mesenteric ischemia”, “visceral angioplasty”, “mesenteric angioplasty”, “mesenteric stents” or their combinations. Eleven reports published between 2002 and 2012 presenting results after surgery intervention were analyzed.
Statistics
All data were documented in an Excel database and final analyses were carried out using SAS v.9.2 (SAS Institute, Cary, NC, USA).
Results
Lesions were treated for the first time in 23 of the 27 patients. Before four cases had been subjected to open surgery due to initial AMS occlusion with reinsertion of the AMS into the aorta below the anatomic origin (2, 1 and 1 at month 1, month 23 and month 34 before, respectively).
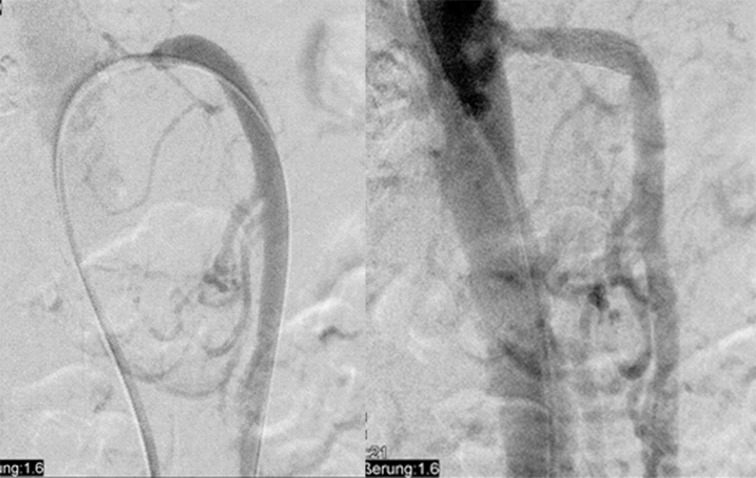
The procedures were performed without clinical complication in all patients. In 25 patients procedures were done by a femoral approach, while in 2 patients a brachial access was used (Fig. 1). Seven patients received pure angioplasty and 20 patients stent-assisted angioplasty using bare metal stents, respectively (17 balloons expandable: RX Herculink Elite Abbot Vascular, USA, range of length 15–24 mm; 3 self-expanding with a length of 60 mm each: Everflex, eV3, Plymouth, USA). Balloon and stent diameter ranged between 5 and 7 mm with a median of 6 mm. Before the procedure all patients was on 100 mg aspirin daily; after the intervention for 4 weeks, 75 mg clopidogrel per day was added for dual antiaggregation without loading dose.
Fig. 1.
Angiograms of the stenosed SMA before and after primary stent placement using a transfemoral approach
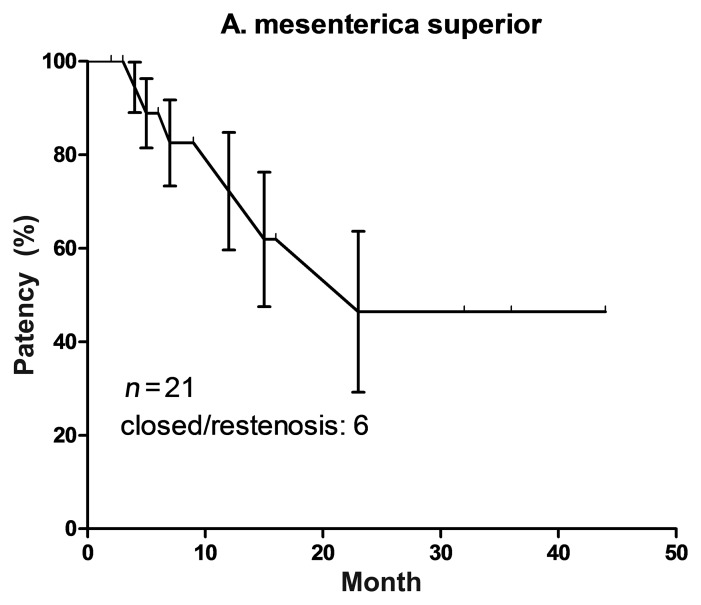
Patients’ median follow-up was 16 months (range 2–60 months). Three patients died 3, 5 and 32 months after the surgery. According to primary physicians or relatives the deaths were not attributed to intestinal ischemia. Five patients underwent reintervention (one early restenosis at day 4 after pure angioplasty with stent placement and 4 because of in-stent restenosis 5 to 23 months after placement). Another patient was treated surgically because of stent occlusion and reoccurrence of abdominal angina 15 months after the intervention. Among these six patients there were two patients who were treated with self-expanding stents of 60 mm length; two received stents of 5 mm diameter only. Primary patency rate is shown as Kaplan–Meier curve in Fig. 2. It gradually decreased below 50% in 24 months. Five patients underwent successful reintervention because of stenosis. Four patients were followed up for another 7 to 27 months without new stenosis. One patient was lost for the follow-up.
Fig. 2.
Kaplan–Meier curve of primary patency after interventional SMA treatment including standard error of primary patency
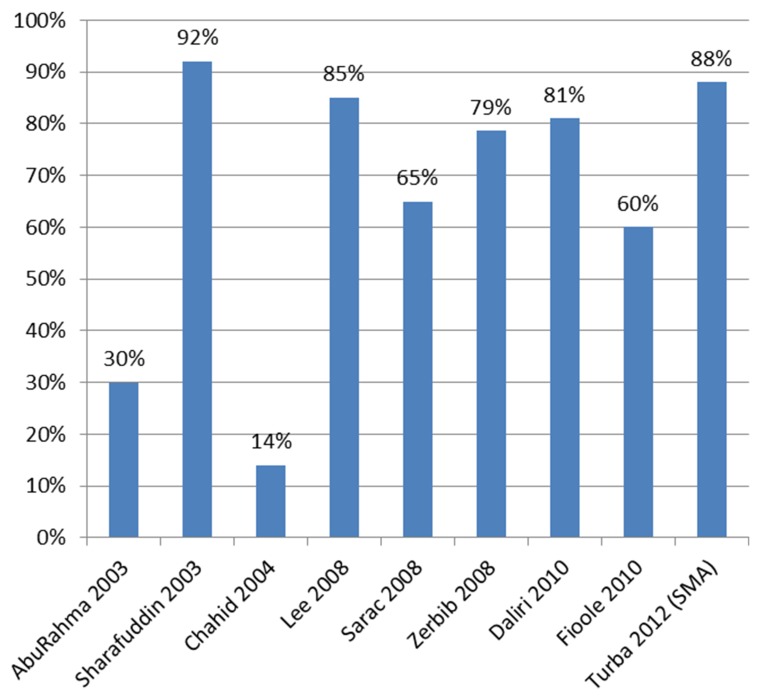
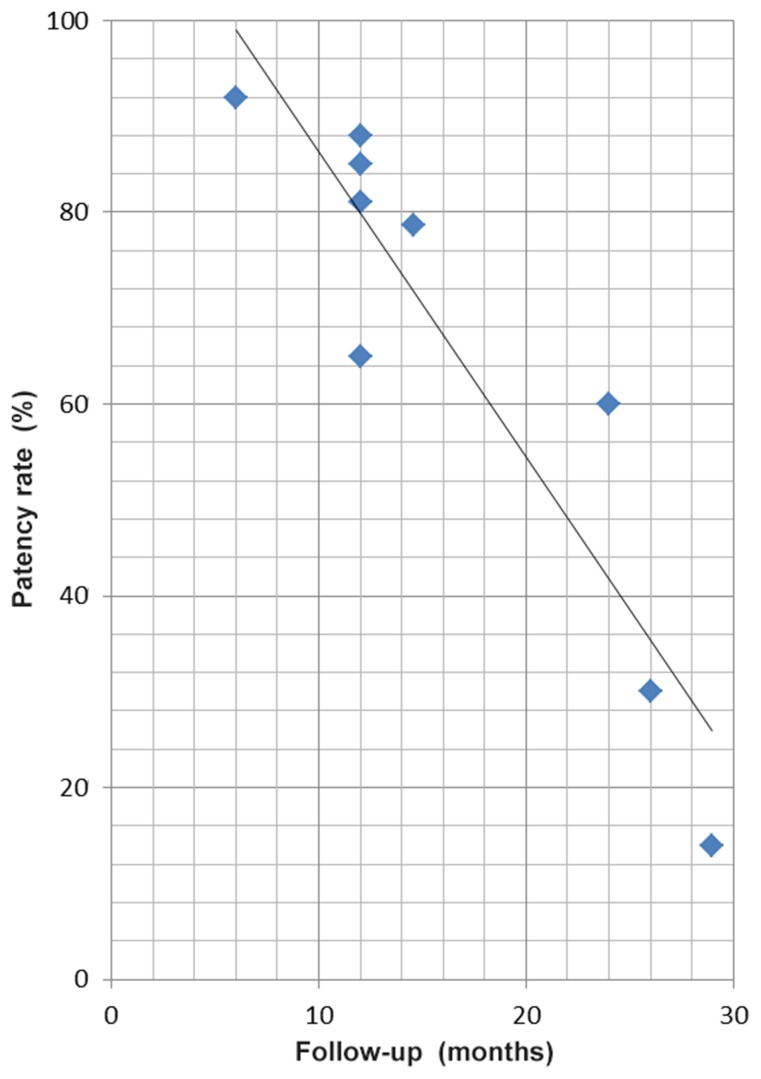
The 11 studies identified in the literature included 429 cases with 196 treatments of the TC, 319 of the SMA and 42 of the IMA (Table I). Most studies included a small number of patients and most patients received stents. In-hospital mortality was low in general, but was 14% (31) and 18% (21) in two centers. Patency rates for CMI treatment were also extracted and are presented in Figs 3 and 4. In general, the patency rates in recent studies are high with up to 88% within 1 year. Data of studies published before 2008 show low patency rates after up to three years. Our two years patency rate of 50% shown in the Kaplan–Meier curve is exactly within the range of patency rates published.
Table I.
Studies reporting interventional SMA treatment
| Author, year, patients | Vessels | Age (y), sex (%) |
Technical (techn.), clinical (clin.) success, morbidity, mortality | Patency rate | Pure PTA / stent |
|---|---|---|---|---|---|
| Pietura, 2002, (n = 6) [17] |
TC 4, SMA 5 | 46–73 y, 5 m (83%) |
100% morbidity/mortality: 0 |
no restenosis after 12–18 months | only one stenting after unsuccessful PTA |
| AbuRahma, 2003, (n = 22) [18] |
TC 12, SMA 8, both 2 |
69.2 (52–88) y, 3 m (14%) |
96%/95% mortality: 0 |
30% after mean 26 months | all PTA/stent |
| Sharafuddin, 2003, (n = 25) [19] |
TC 11, SMA 18 | 66 (41–86) y, 13 m (52%) |
96%/88% morbidity: 10%, 30 d mortality: 4% |
6 months: 92% | all PTA/stent |
| Chahid, 2004, (n = 14) [10] |
TC 3, SMA 8, both 3 |
53 (32–74) y, 6 m (43%) |
100%/100% morbidity: 14%, mortality: 0 |
14% after mean 29 months | 9 : 5 |
| Lee, 2008, (n = 31) [13] |
TC 15, SMA 26 | 70 (43–90) y, 5 m (16%) |
techn.: 97.5% morbidity: 6%, 30 d mortality: 14% |
1 year: 85% no restenosis | |
| Sarac, 2008, (n = 65) [15] |
TC 23, SMA 57, IMA 7 | 70 (44–89) y, 29 m (45%) |
clin.: 85% morbidity: 31%, 30 d mortality: 8% |
1 year: 65% | all PTA/stent |
| Zerbib, 2008, (n = 14) [20] |
TC 2, SMA 12 | 75.4 ± 10.1 y, 8 m (57.2%) |
92.8% morbidity: 21%, 3 mon. mortality: 14.2% |
primary restenosis 21.4% after PTA, pain relief at the end of the study 71.4% | 3 : 11 s |
| Daliri, 2010, (n = 17) [21] |
TC 13, SMA 13 | 65 (31–89) y, 4 m (24%) |
100%/88%mortality: 18% | 1 year: 81%, reintervention rate 30% |
13 : 13 |
| Fioole, 2010, (n = 51) [22] |
TC 30, SMA 24, IMA 6 | 64 ± 11 y, 26 m (51%) |
93%/78% morbidity: 4%, 30 d mortality: 0 |
1 year: 86% 2 years: 60% |
All PTA/stent |
| Jung, 2011, (n = 18) [23] |
TC 6, SMA 16, IMA 1 | 61.5 (43–84) y, 13 m (72%) | 3× recurrence after 6/8/8 months | 94% PTA/stent | |
| Turba, 2012, (n = 166) [24] |
TC 66, SMA 120, IMA 35 | 69 (40–94) y, 54 m (33%) |
97%/88.2% morbidity: 3.6%, 30 d mortality: 3% |
1 year: CT, SMA, IMA 85%, 88%, 84% | 50 : 165 |
Fig. 3.
Patency rates after median follow-up as reported in studies listed in Table I
Fig. 4.
Time plot of the patency rates after median follow-up as reported in the studies shown in Table I
Discussion
Presented data show that endovascular SMA treatment is a suitable and safe procedure in patients suffering from CMI, but long-term results are still limited.
Stenosis of the SMA affects often short areas and is located in the proximal part of the abdominal artery. It is associated with aortic atheroma and can also occur in the presence of aortic aneurysm [1, 5]. Peripheral lesions are rare and described in patients suffering from Takayasu arteritis [8]. In general, complications associated with endovascular treatment include thrombosis, dissection, and embolism that affect renal arteries, legs or peripheral visceral vessels with the risk of acute mesenteric ischemia and death. In rare cases reperfusion hemorrhages have been described [9]. The need of primary or secondary stenting is still under discussion as well as the use of covered stents [10]. It is unknown whether drug eluting stents (DES) can reduce the risk of this complication, while some authors already recommend DES if vessel diameter is below 4–4.5 mm [11].
Both femoral or brachial arteries can be used as a site of access for angioplasty. Some authors prefer brachial arteries, because they observed a lower risk of restenosis when these are used [14, 15].
Angioplasty of the visceral vessels provides good results in the short-term, but is associated with the high risk of early restenosis. Exact numbers vary over study groups. Kougias et al. [12] described a 91% initial technical success with angioplasty. The 30-day mortality with angioplasty is 3.7% (compared to 15.4% after bypass surgery). However, 5-year patency rate is much lower, 32% versus 69% in favor of bypass surgery. Restenosis develops early after angioplasty in the majority of cases [13]. In order to prevent thrombosis complications, dual antiplatelet therapy with aspirin and clopidogrel is widely used after stenting, although no controlled studies analyzed the efficacy of this approach.
In their review Gupta et al. [6] reported 4.4 times longer (95% CI 2.8 to 7.0, p = 0.001) symptom-free survival at 5 years for open versus endovascular surgery. Simultaneously, the complication rate for open versus endovascular surgery was also 3.2 times higher (95% CI 2.5 to 4.2, p = 0.001). Pecoraro et al. [7] focused on safety and reported that at 5 years follow-up, the survivor rate in the endovasular group with 69.41% was statistically not different from the survivor rate in the open surgical group with 65.02%.
Keeping the high restenosis rate in mind all patients should be reassessed regularly – e.g. at months 1, 6 and 12 months, then every 12 months after intervention by obtaining medical history regarding abdominal symptoms, performing physical examination and an imaging technique, preferably duplex ultrasonography. Ultrasonography is useful to identify restenosis, particularly when measurements are compared to those obtained directly after intervention [16].
Duplex ultrasonography is often used as a screening method if image quality in the patient is sufficient. However, the gold standard in visualization of the visceral arteries is still mesenteric angiography that also provides an opportunity to perform immediate intervention. Computed (CTA) or magnetic resonance angiography (MRA) techniques are, however, also available for patients’ assessment [5, 13].
Limitations
As with the published reports the number of patients is limited in our analysis. Differentiation between ostial and non-ostial; and calcified and non-calcified stenosis do not make sense in these small numbers. Patient population was heterogeneous in term of technical procedures, kind of stents placed in vessels and post-interventional anticoagulation procedures. Thus all conclusions derived from the reports cannot be generalizable.
Conclusions
Endovascular treatment can be considered as an alternative in patients with CMI; however, the treatment strategy should always be tailored to the individual needs of the patient.
Funding Statement
Funding sources: None.
Footnotes
Author's contributions: AS – concept and design, writing, critical revision and final approval of the article, literature search; AZ and TM – analysis and interpretation, data collection, statistical expertise; BL – critical revision, final approval of the article, literature search; KK – concept and design, analysis and interpretation, critical revision and final approval of the article, data collection, statistical expertise. All authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest: None.
Contributor Information
Andreas Sundermeyer,
Alexander Zapenko,
Theodoros Moysidis,
Bernd Luther,
Knut Kröger,
References
- 1.Luther B, Meyer F, Nowak T, Kempf U, Krasniqi H. [Chronically progressive occlusive disease of intestinal arteries – Short overview from a vascular surgical perspective] Zentralbl Chir. 2011 Jun;136(3):229–236. doi: 10.1055/s-0031-1271360. [DOI] [PubMed] [Google Scholar]
- 2.Penugonda N, Gardi D, Schreiber T. Percutaneous intervention of superior mesenteric artery stenosis in elderly patients. Clin Cardiol. 2009 May;32(5):232–235. doi: 10.1002/clc.20446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furrer J, Grüntzig A, Kugelmeier J, Goebel N. Treatment of abdominal angina with percutaneous dilatation of an arteria mesenterica superior stenosis. Preliminary communication. Cardiovasc Intervent Radiol. 1980;3(1):43–44. doi: 10.1007/BF02551961. [DOI] [PubMed] [Google Scholar]
- 4.Phipp LH, Scott DJ, Kessel D, Robertson I. Stent implantation for mesenteric bypass graft stenosis. J Endovasc Ther. 2000 Aug;7(4):320–323. doi: 10.1177/152660280000700411. [DOI] [PubMed] [Google Scholar]
- 5.Gibbons CP, Roberts DE. Endovascular treatment of chronic arterial mesenteric ischemia: a changing perspective? Semin Vasc Surg. 2010 Mar;23(1):47–53. doi: 10.1053/j.semvascsurg.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Gupta PK, Horan SM, Turaga KK, Miller WJ, Pipinos II. Chronic mesenteric ischemia: endovascular versus open revascularization. J Endovasc Ther. 2010 Aug;17(4):540–549. doi: 10.1583/09-2935.1. [DOI] [PubMed] [Google Scholar]
- 7.Pecoraro F, Rancic Z, Lachat M, Mayer D, Amann-Vesti B, Pfammatter T, Bajardi G, Veith FJ. Chronic mesenteric ischemia: critical review and guidelines for management. Ann Vasc Surg. 2013 Jan;27(1):113–122. doi: 10.1016/j.avsg.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Loffroy R, Guiu B, Cercueil JP, Krausé D. Chronic mesenteric ischemia: efficacy and outcome of endovascular therapy. Abdom Imaging. 2010 Jun;35(3):306–314. doi: 10.1007/s00261-009-9515-x. [DOI] [PubMed] [Google Scholar]
- 9.Moore M, McSweeney S, Fulton G, Buckley J, Maher M, Guiney M. Reperfusion hemorrhage following superior mesenteric artery stenting. Cardiovasc Intervent Radiol. 2008 Jul;31(Suppl 2):S57–S61. doi: 10.1007/s00270-007-9204-5. [DOI] [PubMed] [Google Scholar]
- 10.Chahid T, Alfidja AT, Biard M, Ravel A, Garcier JM, Boyer L. Endovascular treatment of chronic mesenteric ischemia: results in 14 patients. Cardiovasc Intervent Radiol. 2004 Nov-Dec;27(6):637–642. doi: 10.1007/s00270-004-0225-z. [DOI] [PubMed] [Google Scholar]
- 11.Cardaioli P, Rigatelli G, Zattoni L, Giordan M. Drug-eluting stent for recurrent mesenteric artery in-stent restenosis. J Endovasc Ther. 2007 Oct;14(5):748–751. doi: 10.1177/152660280701400522. [DOI] [PubMed] [Google Scholar]
- 12.Kougias P, Huynh TT, Lin PH. Clinical outcomes of mesenteric artery stenting versus surgical revascularization in chronic mesenteric ischemia. Int Angiol. 2009 Apr;28(2):132–137. [PubMed] [Google Scholar]
- 13.Lee RW, Bakken AM, Palchik E, Saad WE, Davies MG. Long-term outcomes of endoluminal therapy for chronic atherosclerotic occlusive mesenteric disease. Ann Vasc Surg. 2008 Jul-Aug;22(4):541–546. doi: 10.1016/j.avsg.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Kim WT, Ahn SG, Lee JW, Sung JK, Lee SH, Yoon J. Successful recanalization of chronic total occlusion of the superior mesenteric artery by transradial approach. J Zhejiang Univ Sci B. 2010 Aug;11(8):627–630. doi: 10.1631/jzus.B1001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarac TP, Altinel O, Kashyap V, Bena J, Lyden S, Sruvastava S, Eagleton M, Clair D. Endovascular treatment of stenotic and occluded visceral arteries for chronic mesenteric ischemia. J Vasc Surg. 2008 Mar;47(3):485–491. doi: 10.1016/j.jvs.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 16.Abu Rahma AF, Stone PA, Bates MC, Welch CA. Angioplasty/stenting of the superior mesenteric artery and celiac trunk: early and late outcomes. J Endovasc Ther. 2003 Dec;10(6):1046–1053. doi: 10.1177/152660280301000604. [DOI] [PubMed] [Google Scholar]
- 17.Pietura R, Szymańska A, El Furah M, Drelich-Zbroja A, Szczerbo-Trojanowska M. Chronic mesenteric ischemia: diagnosis and treatment with balloon angioplasty and stenting. Med Sci Monit. 2002 Jan;8(1):PR8–PR12. [PubMed] [Google Scholar]
- 18.Aburahma AF, Mousa AY, Stone PA, Hass SM, Dean LS, Keiffer T. Duplex velocity criteria for native celiac/superior mesenteric artery stenosis vs in-stent stenosis. J Vasc Surg. 2012 Mar;55(3):730–738. doi: 10.1016/j.jvs.2011.10.086. [DOI] [PubMed] [Google Scholar]
- 19.Sharafuddin MJ, Olson CH, Sun S, Kresowik TF, Corson JD. Endovascular treatment of celiac and mesenteric arteries stenoses: applications and results. J Vasc Surg. 2003 Oct;38(4):692–698. doi: 10.1016/s0741-5214(03)01030-9. [DOI] [PubMed] [Google Scholar]
- 20.Zerbib P, Lebuffe G, Sergent-Baudson G, Chamatan A, Massouille D, Lions C, Chambon JP. Endovascular versus open revascularization for chronic mesenteric ischemia: a comparative study. Langenbecks Arch Surg. 2008 Nov;393(6):865–870. doi: 10.1007/s00423-008-0355-x. [DOI] [PubMed] [Google Scholar]
- 21.Daliri A, Grunwald C, Jobst B, Szucs-Farkas Z, Diehm NA, Kickuth R, Do DD, Hoppe H. Endovascular treatment for chronic atherosclerotic occlusive mesenteric disease: is stenting superior to balloon angioplasty? Vasa. 2010 Nov;39(4):319–324. doi: 10.1024/0301-1526/a000056. [DOI] [PubMed] [Google Scholar]
- 22.Fioole B, van de Rest HJ, Meijer JR, van Leersum M, van Koeverden S, Moll FL, van den Berg JC, de Vries JP. Percutaneous transluminal angioplasty and stenting as first-choice treatment in patients with chronic mesenteric ischemia. J Vasc Surg. 2010 Feb;51(2):386–391. doi: 10.1016/j.jvs.2009.08.055. [DOI] [PubMed] [Google Scholar]
- 23.Jung YM, Jo YJ, Ahn SB, Son BK, Kim SH, Park YS, Bae JH, Cho YK. Clinical effectiveness of percutaneous angioplasty for acute and chronic mesenteric ischemia: a six case series. Korean J Gastroenterol. 2011 Apr;57(4):243–248. doi: 10.4166/2011.57.4.243. [DOI] [PubMed] [Google Scholar]
- 24.Turba UC, Saad WE, Arslan B, Sabri SS, Trotter S, Angle JF, Hagspiel KD, Kern JA, Cherry KJ, Matsumoto AH. Chronic mesenteric ischaemia: 28-year experience of endovascular treatment. Eur Radiol. 2012 Jun;22(6):1372–1384. doi: 10.1007/s00330-011-2376-z. [DOI] [PubMed] [Google Scholar]