Table 3.
Synthesis of 2-allyltryptophans 3k–o.a
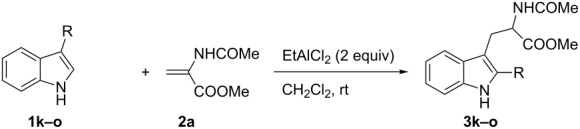 | ||||
| Entry | Indole | Tryptophan | Time (h) | Yield (%)b |
| 1 |
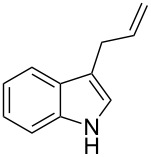 1k |
 3k |
48 | 61 |
| 2 |
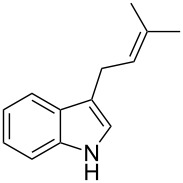 1l |
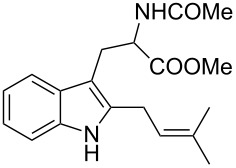 3l |
16 | 86 |
| 3 |
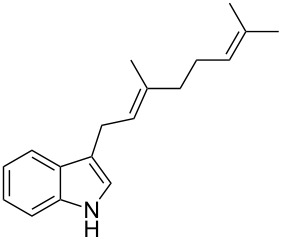 1m |
 3m |
16 | 70 |
| 4 |
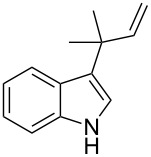 1n |
 3n |
72 | NR |
| 5 |
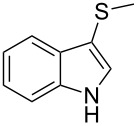 1o |
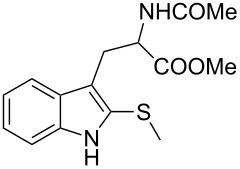 3o |
48 | 68 |
aReaction conditions: 1k–o (0.25 mmol), 2a (0.3 mmol), EtAlCl2 (0.5 mmol), CH2Cl2 (2.5 mL), rt. bYields of the isolated products after column chromatography. NR, no reaction.
