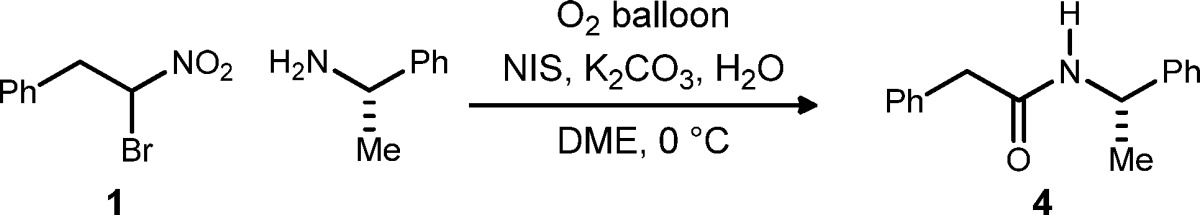
Table 1. Investigation of NIS Reagent Loading Under Anaerobic and Aerobic Conditions: Catalyzed Umpolung Amide Synthesis.
| entrya | O2b | NIS amount (mol %) | yield (%)c |
|---|---|---|---|
| 1 | no | 100 | 69 |
| 2 | no | 75 | 52 |
| 3 | no | 50 | 36 |
| 4 | no | 30 | 25 |
| 5 | no | 20 | 20 |
| 6 | no | 10 | 20 |
| 7 | no | 0 | 19 |
| 8 | yes | 20 | 76 |
| 9 | yes | 10 | 73 |
| 10 | yes | 5 | 76 |
| 11 | yes | 1 | 55 |
| 12e | yes | 0 | 51 |
| 13e | yesd | 5 | 69 |
Reactions are 0.2 M in nitroalkane and employ 1.2 equiv of amine and 5 equiv of H2O and run for 24 h. Under anaerobic conditions (entries 1–7), NIS was added once the nitroalkane was no longer visible by TLC, indicating that the nitronate predominated (see Supporting Information).
All solutions were first degassed (freeze–pump–thaw cycles), and entries not using oxygen were performed under an argon atmosphere (balloon), whereas entries using oxygen were performed under an oxygen atmosphere (balloon).
Isolated yield.
Open to air.
48 h reaction time.
