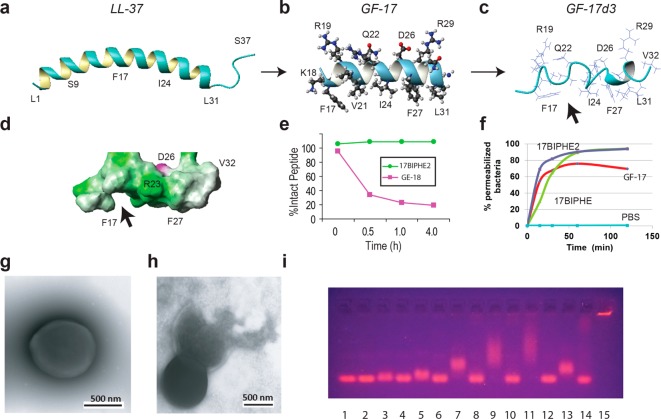
Figure 1.
Structure-based design of potent peptides to combat the ESKAPE pathogens. Shown are NMR structures of human LL-37 (A); its peptides GF-17 (B) and GF-17d3 (C and D) bound to membrane-mimetic micelles;10,15,18 peptide degradation kinetics (E);19 flow cytometry of S. aureus USA300 after treatments with 40 μM GF-17, 17BIPHE, or 17BIPHE2 peptides (F);18 transmission electron microscopy images of S. aureus USA300 before (G) and after (H) peptide treatment;14 and DNA retardation (I) by the peptides in Table 1. Each peptide was evaluated by a 1% agarose gel (casted with 1 μg/mL ethidium bromide) at two concentrations, first at 6 and then 12 μM (lanes 1: 75 ng pUC19 DNA plasmid itself; 2 and 3: 17F2; 4 and 5: 17mF-F; 6 and 7: 17F-Naph; 8 and 9: 17mF-Naph; 10 and 11: 17Naph-mF; 12 and 13: 17BIPHE; 14 and 15: 17BIPHE2). Structural images were generated using MOLMOL.29