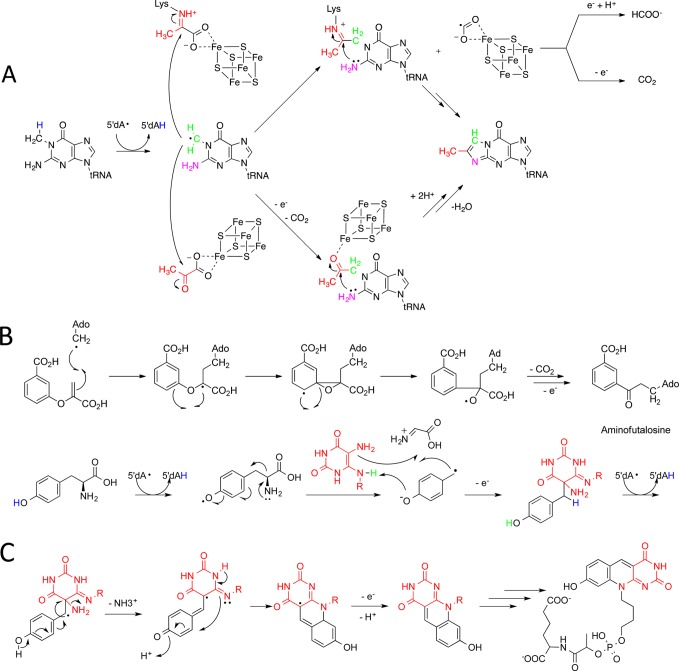
Figure 3.
(A) Two mechanisms have been proposed for the conversion of N-methylguanosine to 4-demethylwyosine catalyzed by Tyw1. (top) A lysine stabilizes intermediates by forming a Schiff base with pyruvate; the auxiliary cluster functions as a redox center.43 (bottom) Coordination of the auxiliary cluster promotes the reaction of the substrate radical with pyruvate facilitates cyclization.44 (B) Proposed mechanism for aminofutalosine synthesis catalyzed by MqnE. The key first step involves a radical Michael addition of Ado• to the chorismate-derived cosubstrate; subsequent rearrangement and oxidative decarboxylation produces aminofutalosine. (C) Proposed mechanism for the reaction of tyrosine and diaminouracil catalyzed by F0 synthase. The mechanism involves the abstraction of hydrogen from tyrosine and the release of a dehydroglycine, producing a reactive 4-oxidobenzyl radical, which adds to diaminouracil. A second round of radical chemistry initiates formation of the tricyclic deazaflavin, F0, that in further steps is converted to F420.