Table 1.
Pd-Catalysed cyclisations of unsaturated polyols.
 | ||||
| Entry | Substrate | Reaction conditionsa | Product(s) | Yield (%) |
| 1 |
 11 |
Method A |
 44 |
79 [23] |
| 2 |
 12 |
Method A Method C |
 45 |
63 40 |
| 3 |
 13 |
Method A | Complex mixture | |
| 4 |
 33 |
Method A Method B |
 51 + 52 |
15 (51), 25 (52) 65 (52) |
| 5 |
 E-21 |
Method A |
 46 |
30 |
| 6 |
 24–26 |
Method A |
 47 + 48 |
66 (47/48, 5:3) |
| 7 |
 28 |
Method A |
 49 + 50 |
54 (49/50, 5:3) |
| 8 |
 37 |
Method A Method B Pd(PPh3)4b |
 56 + 57 |
70 (56/57, 1:3) 69 (56/57, 1:3) 84 (56/57, 1:3) |
| 9 |
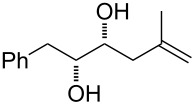 35 |
Method A |
 53 |
33 |
| 10 |
 30 |
Method A Method B |
 54 + 55 |
38 (54), 35 (55) 70 (55) |
| 11 |
 threo-9 |
Method B |
 58 |
78 |
aMethod A: PdCl2 (0.1 equiv), CuCl2 (3 equiv), NaOAc (3 equiv), AcOH, rt.; method B: PdCl2(MeCN)2 (0.1 equiv), BuLi (2 equiv), CuCl2 (3 equiv), LiCl (3 equiv), THF, rt; method C: Pd(OAc)2 (0.1 equiv), PhI(OAc)2 (2 equiv), Me4N+Cl− (1 equiv), NaOAc (1 equiv), AcOH, rt. bLit. [36–38] Pd(PPh3)4 (0.1 equiv), THF, rt.
