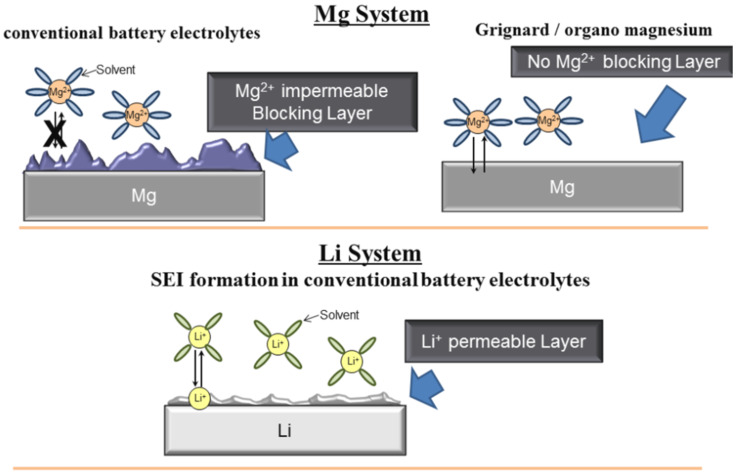
Figure 1.
Schematic depicting a simplified image of metal–electrolyte interfaces for magnesium and lithium metals. The magnesium metal case; unlike the lithium, experiences a blocking layer formation when exposed to conventional electrolytes, i.e., ionic salts and polar solvents. No Mg passivation (bare Mg) occurs in ethereal organo-magnesium electrolytes.