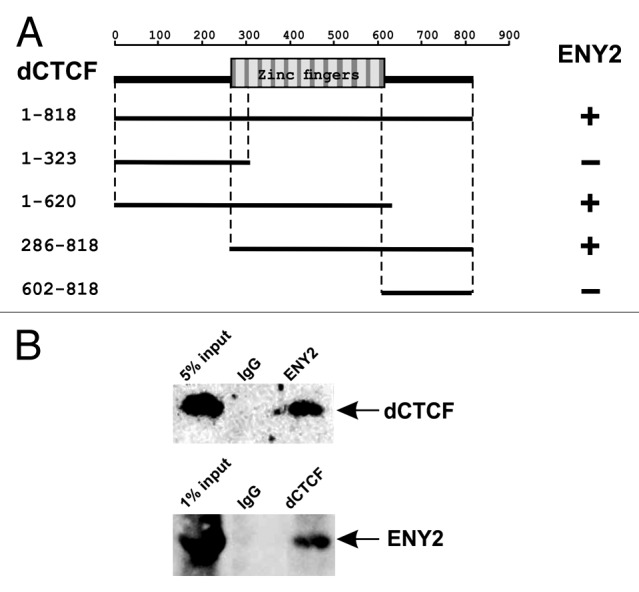
Figure 1. Interaction of dCTCF and ENY2 proteins. (A) ENY2 interacts with zinc-finger domain of dCTCF in the yeast two-hybrid assay. The scheme shows the structure of the full-length dCTCF protein and the polypeptides tested. The plus and minus signs indicate relatively strong interaction and the absence of interaction, respectively. Different fragments of dCTCF were individually fused to the C terminus of the GAL4 activating domain and analyzed for the interaction with E(y)2 fused to the DNA-binding domain of GAL4. All dCTCF fragments were tested for the absence of interaction with GAL4. (B) Co-immunoprecipitation of dCTCF and ENY2 proteins from S2 cell extract. The immunoprecipitated complexes were washed with 300 mM, 500 mM and 150 mM NaCl-containing buffers before resolving them by SDS-PAGE for western blot analysis with the indicated antibodies.
