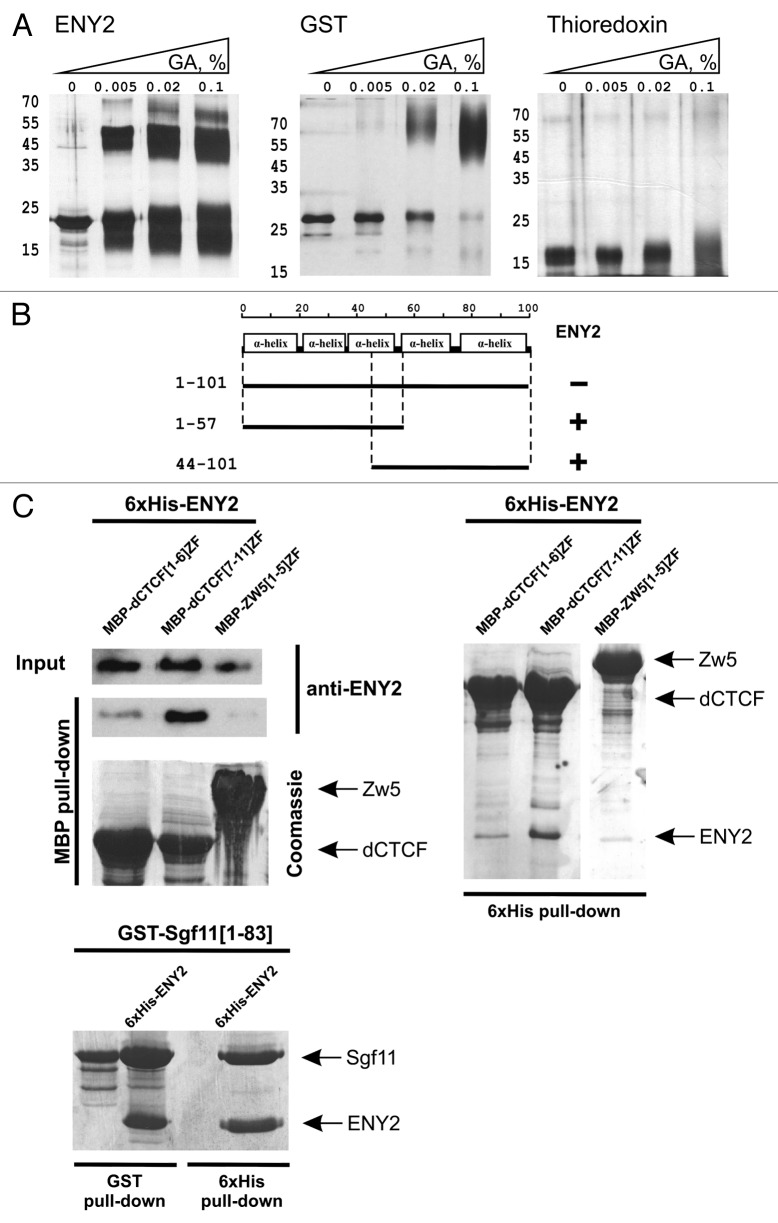
Figure 2. Tests for the interaction between ENY2 and dCTCF in vitro. (A) Cross-linking of ENY2 by incubation with increasing concentration of glutaraldehyde (GA). Proteins were separated in 5–12% gradient polyacrylamide gels and visualized by silver staining (for experimental details, see Materials and Methods). GST was used as a positive control of dimerization. Thioredoxin used as a negative control is shown presented as a monomer molecule. (B) ENY2 interacts with parts of ENY2 in the yeast two-hybrid assay. (C) ENY2 interacts with zinc fingers 7–11 of dCTCF in co-expression assay. Indicated MBP-fused zinc-finger domains were co-expressed with 6 × His-ENY2. The results of 6 × His (stained with Coomassie) and MBP (stained with Coomassie/anti-ENY2 antibodies) pull-down assays are shown. “Input” refers to bacterial lysate. For Sgf11–ENY2, the results of GST and 6 × His pull down assays stained with Coomassie are shown. Arrows indicate positions of MBP-fused zinc fingers of dCTCF or Zw5, GST-fused N-terminus of Sgf11, and 6 × His-ENY2. The zinc-finger domain of Zw5 protein (negative control) displayed no direct interaction with ENY2. The Sgf11 protein (positive control) interacted with ENY2.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.