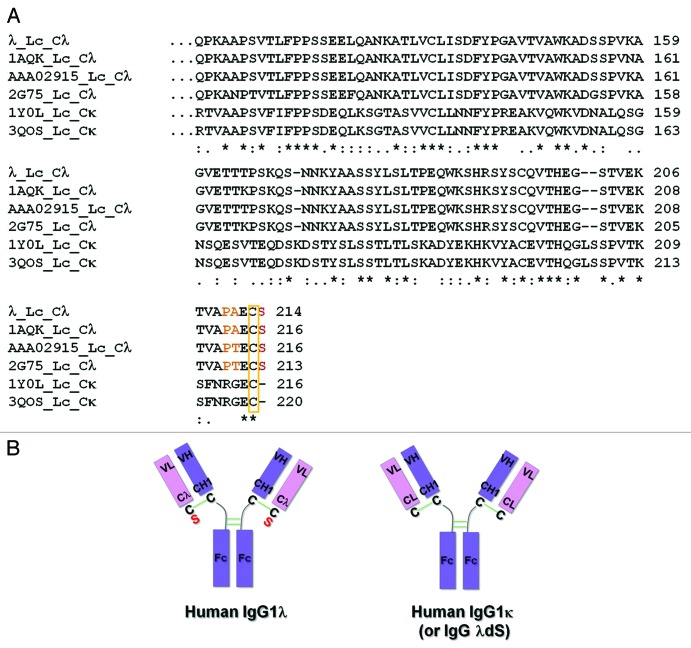
Figure 1. Differences between representative human IgG1λ and IgG1κ light chain constant regions. (A) Human IgG lambda light chain constant domain (Cλ) sequences from λ, PDB:1AQK, PDB:2G75 and GenBank:AAA02915, and kappa light chain constant domain (Cκ) sequences from PDB:1Y0L and PDB:3QOS are aligned and compared. In comparison to Cκ sequences, all Cλ domains terminate with an extra serine (red) following the conserved cysteine residue (yellow rectangle), which forms the inter-chain disulfide bond with the heavy chain. Amino acids which differ from the corresponding Cκ positions and are immediately before the cysteine in Cλ sequences are highlighted in orange. (B) Cartoon illustration of human IgG1λ and human IgG1κ (or human IgG1λ serine deletion – dS) structures. The extra serine residue trailing the λLc is highlighted in red. All the interchain disulfide bonds are drawn as green solid lines.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.