Introduction
Impaired Glucose Tolerance (IGT), impaired fasting glucose (IFG), and metabolic syndrome are considered precursors to type 2 diabetes (T2D) mellitus.1 Endothelial dysfunction is also associated with increased risk for diabetes and is directly linked to insulin resistance2 and hyperglycemia.
While pharmacotherapy with such drugs as metformin, acarbose, orlistat, and thiazolidinediones can reduce risk of T2D,3 their cost and potential adverse effects can be objectionable to patients who do not yet have an actual disease.4 Intensive diet and lifestyle change can play an important role in diabetes prevention5 though adherence to these regimens is often difficult.6
The micronutrient chromium (Cr) is of interest in this regard as a potential means of improving glucose tolerance7,8 by reducing insulin resistance.9 Chromium picolinate is widely marketed to the public with diverse health claims pertaining to glucose metabolism, insulin action, muscle mass, weight control, and diabetes prevention.10 In 2002, estimated sales of chromium-based supplements was $85 million (USD).11 Indeed, one of the more common nutrition-related questions posed by patients with or at risk for diabetes to practicing endocrinologists concerns the effectiveness of chromium.
To assess the efficacy of this popular nutritional supplement, we performed a randomized controlled trial designed to investigate the effects of daily chromium picolinate supplementation for six months at two dose levels on serum measures of glucose tolerance and insulin sensitivity. Because of the association of derangements in these metabolic abnormalities with endothelial dysfunction, brachial artery reactivity was also assessed before and after therapy.
Research Design and Methods
Participants
Patients enrolled were aged 18 years of age or older identified to have either 1) IGT 2) IFG or 3) metabolic syndrome.
IGT was diagnosed by American Diabetes Association (ADA) guidelines1 requiring the following two criteria: 1) Plasma glucose two hours (2hrPG) after consuming 75 g of glucose is at least 140 mg/dl but below 200 mg/dl and 2) fasting plasma glucose (FPG) level is less than 126 mg/dl.
IFG was diagnosed using the ADA criteria of a FPG concentration of 100 mg/dl or greater, but less than 126 mg/dl.1
Metabolic syndrome was diagnosed using NCEP ATP III criteria,12 requiring the presence of three of the following five criteria: waist circumference >102 cm in men or >88 cm in women; triglyceride level ≥150 mg/dl; HDL-C <40 mg/dl in men or <50 mg/dl in women; blood pressure >130/>85 mm Hg; and FPG ≥100 mg/dl.
Subjects were excluded if they were diabetic (FPG >126 mg/dl; 2hrPG >200 mg/dl). Other exclusion criteria included self-reported hospitalization for treatment of cardiovascular disease six months prior to enrollment, serum creatinine >2.0 mg/dl at baseline, self-reported pancreatitis, recent or significant abdominal surgery, pregnancy and/or intention to become pregnant during the study, polycystic ovarian syndrome or irregular menses, and use of chromium supplements less than one month prior to screening.
Subjects taking drugs thought to affect glucose metabolism and/or endothelial function were excluded (glucocorticoids, antineoplastic agents, psychoactive agents, and bronchodilators.) Subjects taking antihypertensive drugs and lipid-lowering agents were allowed to participate provided that doses were stable for three months prior to enrolment.
Ethical and Safety Considerations
The study protocol and consent form were approved by the Griffin Hospital (Derby, CT) Institutional Review Board and the Yale University (New Haven, CT) Human Investigation Committee and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained, and all subjects received monetary compensation for their participation. For safety monitoring, unblinded subject treatment assignment was maintained by a Data and Safety Monitoring Board (DSMB).
Study Design and Interventions
This study was a randomized, double-blind, placebo controlled, modified crossover clinical trial to investigate the effects of daily chromium supplementation for six months at two dose levels (500 mcg and 1000 mcg of chromium picolinate per day) on serum measures of insulin sensitivity and glucose tolerance in adults with IGT, IFG, and metabolic syndrome.
The study used a modified cross-over (Latin square) design encompassing both paired (crossover) and unpaired comparisons with statistical methods and sample size tailored to serve both purposes (see Statistical Analysis).
The study was designed and powered to compare the six-month effects of 500 mcg to 1000 mcg of chromium on insulin resistance (HOMA-IR), which provided adequate power (see Statistical Analysis) to detect a change in 2hrPG, and endothelial function. Effects of chromium at each dose were compared to placebo as a paired (crossover) comparison after six months of use. Because the time required for chromium to wash out fully is unknown, a post-treatment phase of six months was incorporated into the design following 12-months of intervention and placebo (see Figure 1).
Figure 1.
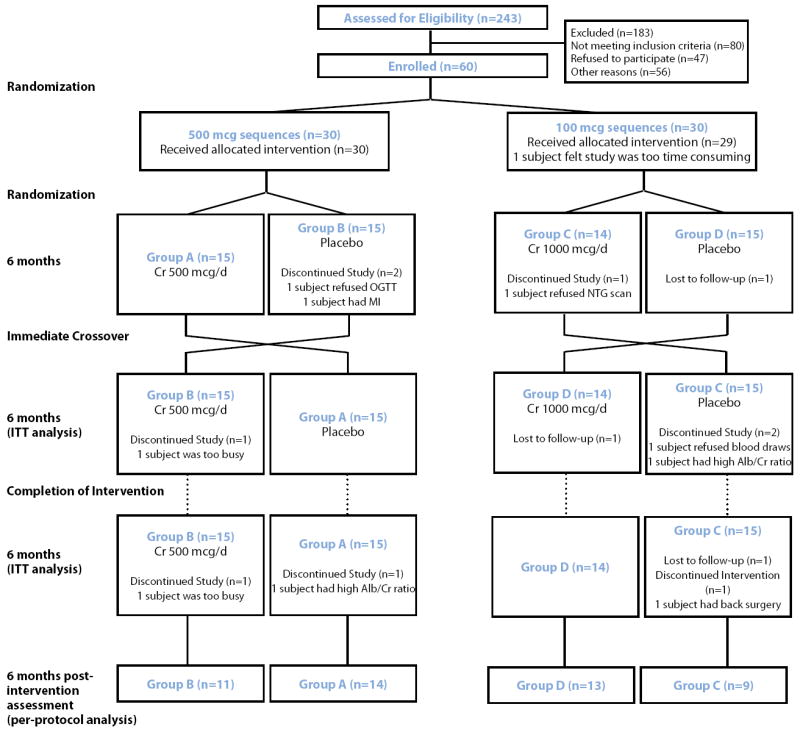
Study Design and Flow Diagram.
Subjects meeting eligibility criteria were randomly assigned to 500 mcg/day or 1000 mcg/day of elemental chromium as chromium picolinate and then further randomized chromium/placebo or placebo/chromium sequences. After completing the initial six-month period, subjects immediately crossed-over to the alternate assignment (see Figure 1). All investigators and participants were blinded to treatment assignment.
The two dosages of chromium (500 mcg or 1000 mcg/day) and placebo capsules came in the form of capsules similar in shape, size, and appearance, donated by Nutrition 21, Inc (Purchase, NY). Placebo capsules were indistinguishable from those containing chromium. Supplying pharmacy personnel encoded the treatment supplements and matching placebos.
Outcome Measures
The primary outcome measures were serum insulin, HOMA-IR, 2hrPG, FPG, and 2-hour post-OGTT insulin assessed after six months of chromium use. Insulin resistance was calculated using the Homeostasis Model Assessment Insulin Resistance (HOMA-IR) by using the equation, HOMA-IR = fasting plasma insulin (mU/ml) × FPG (mmol/l)/22.5.3
Secondary outcome measures included weight, waist circumference, body-mass index (BMI), blood pressure, endothelial function as assessed by flow-mediated dilatation (FMD) of the brachial artery, blood glycohemoglobin (HbA1c), total serum cholesterol, serum HDL, serum LDL, serum triglycerides, and urinary microalbumin-to-creatinine ratio.
Laboratory measures including FPG, 2hrPG, HbA1c, lipid panel, and urinary markers were collected and analyzed at Griffin Hospital (Derby, CT) using standard procedures at each visit. Insulin concentrations were measured at the Yale Center for Clinical Investigation Core Laboratory (New Haven, CT).
Statistical Analysis
A sample size of 60 subjects, allowing for 20% attrition and nonadherence, was predicted to provide 90%8 power to detect a minimal difference of 9.5% in HOMA sensitivity between the 500 mcg and 1000 mcg arms (α = 0.05). A standard deviation of 10.1 was used based on previous literature.13 This sample size also provided >80% power to detect a change of 15% in 2hrPG and 0.5% in FMD between the 500 mcg and 1000 mcg groups at six months.
Analysis was by intention-to-treat; six-month post-intervention analysis was conducted on those having completed six-month assessments (primary endpoint) in the crossover design. Missing individual data were addressed by the last observation carried forward method.
Results
Of the 243 persons screened for eligibility, 80 did not meet eligibility criteria, 47 refused to participate, and 56 were not randomized for other reasons (see Figure 1). Sixty subjects were ultimately randomized to 500 mcg (n=30) or 1000 mcg (n=30) sequences. One subject in the 1000 mcg sequence withdrew consent after randomization because of time commitment. Of the 59 subjects enrolled, 56 completed six-month assessments (primary endpoint) and 50 subjects completed the six-month post-intervention assessment (see Figure 1). Spot checks of pill counts were done to assess compliance; 92% of our sample consumed over 80% of assigned capsules.
Subjects ranged in age from 31 to 88 years, the mean (SD) age of the participants was 56.9 (12.1) years; 38 (64%) were female. Additional demographic data are provided in Table 1. At baseline, 18 (64%) of subjects in the 500 mcg arm and 20 (74%) of subjects in the 1000 mcg arm were insulin resistant by HOMA-IR (> 3.0)14 (p=0.43). Furthermore, 14 subjects (47%) in the 500 mcg arm and 19 subjects (66%) in the 1000 mcg arm had metabolic syndrome by NCEP ATP III criteria 12 (p=0.19).
Table 1.
Baseline characteristics of 500 mcg and 1000 mcg arms (mean ± SD unless noted).
| Variable | Groups A and B 500 mcg (n=30) | Groups C and D 1000 mcg (n=29) | p-value |
|---|---|---|---|
|
| |||
| Gender | |||
| Male | 11 (36.7%) | 10 (34.5%) | 1.00 |
|
| |||
| Age | |||
| Years | 54.7 ± 10.6 | 59.3 ± 13.3 | 0.15 |
|
| |||
| Anthropometric Measures | |||
| Weight (lb) | 201.1 ± 30.6 | 203.2 ± 50.9 | 0.84 |
| Waist Circumference (cm) | 107.2 ± 7.9 | 110.4 ± 15.8 | 0.41 |
| BMI (kg/m2) | 33.4 ± 6.1 | 32.7 ± 5.6 | 0.64 |
|
| |||
| Metabolic Syndrome (1) | |||
| Yes | 14 (46.7%) | 19 (65.5%) | 0.19 |
|
| |||
| Blood Pressure | |||
| Systolic (mm Hg) | 129.3 ± 12.3 | 129.6 ± 14.4 | 0.93 |
| Diastolic (mm Hg) | 75.6 ± 10.7 | 73.4 ± 12.5 | 0.79 |
|
| |||
| Lab Values | |||
| Fasting Plasma Insulin (μU/ml) | 15.6 ± 8.1 | 18.2 ± 11.6 | 0.33 |
| 2-hour OGTT Insulin (μU/ml) (3) | 61.0 (18,226) | 77.5 (14,483) | 0.32 |
| FPG (mg/dL) | 103.8 ± 10.8 | 98.9 ± 9.0 | 0.06 |
| 2-hour OGTT Glucose (mg/dL) | 131.8 ± 41.9 | 125.8 ± 30.0 | 0.53 |
| HOMA-IR (2) | 4.0 ± 2.0 | 4.4 ± 2.7 | 0.54 |
| Whole blood HbA1c (%) | 5.7 ± 0.4 | 5.7 ± 0.5 | 0.90 |
| Serum total Cholesterol (mg/dL) | 187.8 ± 26.5 | 185.0 ± 36.4 | 0.73 |
| Serum HDL (mg/dL) | 51.7 ± 11.6 | 45.0 ± 11.6 | 0.03 |
| Serum LDL (mg/dL) | 112.0 ± 26.5 | 116.2 ± 29.2 | 0.56 |
| Serum Triglycerides (mg/dL) | 120.1 ± 49.3 | 120.5 ± 48.2 | 0.98 |
| Urine microalbumin (3) | 5.6 (0.0, 90.1) | 7.1 (0.0,57.8) | 0.24 |
| Serum creatinine (mg/dL) | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.53 |
|
| |||
| Endothelial function | |||
| FMD Pre OGTT (% Δ) | 7.8 ± 3.9 | 9.5 ± 4.7 | 0.15 |
| FMD Pre OGTT Nitro (% Δ) | 15.3 ± 5.7 | 16.2 ± 5.9 | 0.56 |
| FMD Post OGTT (% Δ) | 9.5 ± 4.5 | 10.2 ± 5.0 | 0.56 |
| SARM Pre OGTT (4) | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.50 |
| SARM Post OGTT (4) | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.42 |
Metabolic syndrome as defined by NCEP-ATP III criteria.
Homeostasis Model Assessment Insulin Resistance (HOMA-IR) by using the mathematical approximation, HOMA-IR = fasting plasma insulin (mU/mL) × fasting plasma glucose (mmol/L)/22.5.
Values are expressed as median (minimum, maximum).
SARM = stimulus-adjusted response measure.
Primary Outcomes: No changes were observed in FPG, 2hrPG levels, fasting or 2-hour post-OGTT insulin levels, or in HOMA-IR as compared to placebo after six months of chromium use in either the 500 mcg and 1000 mcg arms (see Table 2).
Table 2.
Changes from baseline values in 500 mcg and 1000 mcg arms after six months (mean change with 95% CI).
| Variable | Group A and B 500 mcg (n=30) | Group A and B Placebo (n=30) | Group C and D 1000 mcg (n=29) | Group C and D Placebo (n=29) |
|---|---|---|---|---|
|
| ||||
| Anthropometric Measures | ||||
| Weight (lb) | -0.1 (-3.1 to 2.8) | -1.4 (-4.3 to 1.6) | 2.6 (-0.5 to 5.7) | 0.4 (-2.7 to 3.5) |
| Waist Circumference (cm) | -0.1 (-2.1 to 1.9) | 0.9 (-1.1 to 2.9) | 0.1 (-2.7 to 2.8) | 1.6 (-1.2 to 4.3) |
| BMI (kg/m2) | -0.1 (-0.6 to 0.5) | -0.3 (-0.8 to 0.2) | 0.4 (-0.1 to 0.9) | -0.0 (-0.6 to 0.5) |
|
| ||||
| Blood Pressure | ||||
| Systolic (mm Hg) | 1.3 (-3.3 to 5.8) | -1.2 (-5.7 to 3.3) | 1.3 (-3.2 to 5.7) | 4.6 (0.2 to 9.0) |
| Diastolic (mm Hg) | 2.8 (-0.6 to 6.1) | 3.4 (-0.1 to 6.8) | 0.1 (-3.0 to 3.3) | 1.6 (-1.6 to 4.7) |
|
| ||||
| Lab Values | ||||
| Fasting Plasma Insulin (μU/ml) | 1.3 (-1.0 to 3.5) | -1.0 (-3.2 to 1.2) | 0.7 (-1.6 to 3.0) | -0.4 (-2.7 to 1.9) |
| 2-hour OGTT Insulin (μU/ml) | -1.0 (-11.6 to 9.6) | -7.4 (-18.0 to 3.1) | 6.3 (-28.5 to 41.2) | 5.1(-29.7 to 39.9) |
| FPG (mg/dL) | -1.0 (-3.9 to 1.9) | -2.8 (-5.7 to 0.1) | -0.3 (-3.8 to 3.1) | -0.5 (-3.9 to 3.0) |
| 2-hour OGTT Glucose (mg/dL) | 0.8 (-13.2 to 14.1) | -0.1 (-14.1 to 13.9) | -2.9 (-15.0 to 9.2) | 5.7 (-6.4 to 17.8) |
| HOMA-IR (1) | 0.3 (-0.4 to 1.1) | -0.4 (-1.1 to 0.4) | 0.2 (-0.4 to 0.8) | -0.1 (-0.7 to 0.5) |
| % Δ AHOMA-IR (1) | 7.1 (-6.7 to 21.0) | -6.9 (-20.8 to 6.9) | 5.8 (-8.8 to 20.2) | 7.1 (-7.5 to 21.6) |
| Whole blood HbA1c (%) | 0.1 (0.0 to 0.2) | 0.1 (0.0 to 0.2) | 0.0 (-0.1 to 0.1) | 0.1 (-0.0 to 0.2) |
| Serum total Cholesterol (mg/dL) | -2.9 (-11.0 to 5.1) | -6.8 (-14.9 to 1.2) | -3.1 (-12.5 to 6.3) | 2.4 (-7.0 to 11.8) |
| Serum HDL (mg/dL) | 1.9 (-0.1 to 4.0) | 0.6 (-1.4 to 2.6) | 3.1 (-2.6 to 8.9) | 0.3 (-5.4 to 6.0) |
| Serum LDL (mg/dL) | -6.4 (-13.2 to 0.4) | -7.1 (-13.9 to -0.3) | -5.9 (-14.6 to 2.8) | 0.03 (-8.7 to 8.8) |
| Serum Triglycerides (mg/dL) | 8.7 (-7.5 to 24.9) | -0.9 (-17.1 to 15.4) | -2.9 (-17.1 to 11.2) | 10.4 (-3.8 to 24.5) |
| Urine microalbumin | -1.4 (-3.9 to 1.2) | -2.5 (-5.1 to 0.1) | 1.2 (-2.8 to 5.2) | 1.6 (-2.4 to 5.6) |
| Serum creatinine (mg/dL) (μU/ml) | 0.1 (-0.0 to 0.0) | 0.0 (-0.0 to 0.0) | 0.0 (-0.0 to 0.0) | 0.0 (-0.0 to -0.0) |
|
| ||||
| Endothelial Function | ||||
| FMD Pre OGTT (% Δ) | 1.0 (-0.2 to 2.3) | 1.0 (-0.3 to 2.7) | -0.1 (-1.1 to 1.4) | -0.2 (-1.1 to 1.5) |
| FMD Pre OGTT Nitro (% Δ) | 1.2 (-1.0 to 3.5) | 0.1 (-2.1 to 2.4) | 0.1 (-1.8 to 1.9) | -0.7 (-1.1 to 2.6) |
| FMD Post OGTT (% Δ) | 0.9 (-0.5 to 2.4) | 0.1 (-1.3 to 1.6) | -1.6 (-3.0 to -0.2) | -0.4 (-1.8 to 1.0) |
| SARM Pre OGTT (2) | 0.0 (-0.0 to 0.0) | 0.0 (-0.0 to 0.0) | 0.0 (-0.0 to 0.0) | 0.0 (-0.0 to 0.0) |
| SARM Post OGTT (2) | -0.0 (-0.1 to 0.1) | 0.0 (-0.0 to 0.1) | 0.0 (-0.0 to 0.0) | 0.0 (-0.0 to 0.0) |
Homeostasis Model Assessment Insulin Resistance (HOMA-IR) by using the mathematical approximation, HOMA-IR = Fasting plasma insulin (mU/mL) × fasting plasma glucose (mmol/L)/22.5.
SARM = stimulus-adjusted response measure.
Secondary Outcomes: Six months of supplementation with chromium (500 mcg or 1000 mcg) was not associated with any significant (p>0.05) changes in HbA1c, weight, waist circumference, BMI, blood pressure, total cholesterol, HDL, LDL, triglycerides, or urine microalbumin compared to placebo. A modest change in endothelial function at six months was noted in comparing the 500 mcg arm to the 1000 mcg arm; endothelial function improved in the former group (+0.9% (-0.5 to 2.4) vs. -1.6% (-3.0 to -0.2) in the latter group at 2-hour post OGTT), of uncertain significance, given the lack of other changes in this parameter across groups (see Table 2) and the inverse dose-response. There were no other significant changes observed at the six-month post-intervention assessment on all remaining outcome measures.
No significant changes were observed in any of the primary or secondary outcome measures after controlling for age, gender, BMI at baseline, metabolic syndrome, treatment sequence, and treatment assignment (p>0.05).
Four subjects developed T2D during the course of the study (FPG >126 mg/dl or 2hrPG >200 mg/dl). All four diabetic subjects were in the 500 mcg arm, though two were in the placebo-first sequence while two were in the chromium-first sequence at the time of diagnosis. No serious adverse events were reported.
Discussion
In this randomized prospective study involving adult patients at risk for diabetes, chromium supplementation, at two dosing levels, had no substantive effect on any direct measure of glucose metabolism or indirect measures of insulin action. Chromium therefore appeared ineffective on markers thought to be related to the development of T2D in these high risk subjects. No differences were seen after six months of active treatment vs. placebo, nor after a six-month post-intervention assessment. The single statistically-significant improvement in endothelial function between subjects in the 500 mcg arm compared to the 1000 mcg arm is likely a statistical artifact or random finding due to multiple comparisons, inconsistency with a dose-response effect, and lack of corroboration seen in the stimulus-adjusted response measure (SARM). In prior work, including our own,15-18 we have detected a significant improvement in endothelial function (as flow-mediated dilatation) with a ‘cardiac risk modification’ strategy.19 One proposed explanation is that endothelial function may aggregate the effect of numerous biomarkers of circulatory health. Accordingly, the impact of a single intervention may be amplified when endothelial function is measured. Thus a positive effect of chromium on endothelial function might have suggested a benefit too subtle to capture with our relatively more crude metabolic measures. However, the absence of any such effect buttresses our conclusion that chromium was without substantial benefit in this population.
Our results are congruent with a recent study performed in our lab finding no effect of 1000 mcg of chromium picolinate on weight loss and adiposity in 80 overweight individuals.20 Other recent trials (see Table 3) have found similar results. In a randomized trial of 63 persons with metabolic syndrome, Iqbal et al. found no effect of 1000 mcg of chromium picolinate on insulin sensitivity, glucose metabolism, body weight, serum lipids, or measures of inflammation and oxidative stress.21 Gunton et al. found no changes in 1-and 2-hour glucose tolerance, FPG, fasting insulin, HOMA-IR, and lipid measures in a 3-month RCT using 800 mcg of chromium in a randomized controlled trial in 40 subjects with impaired glucose tolerance.22 Earlier studies had shown positive effects of chromium, but these involved smaller numbers of subjects7 and shorter treatment durations.8 Other prior studies suggested that the primary factor for a clinical response to chromium supplementation is insulin resistance.23-25 In subjects with T2D using sulfonylurea agents, Martin24 et al. demonstrated that chromium picolinate improves insulin sensitivity, glucose control and attenuates body weight and visceral fat compared to placebo. The discrepancy between our results and those of Martin et al. may possibly be explained by differences in the study populations, with ours not including patients with diabetes or by the differential measures of insulin sensitivity used in the two studies. HOMA-IR is predominately affected by hepatic sensitivity, while the hyperinsulinemic euglycemic clamp technique employed by Martin et al. predominately assesses peripheral insulin sensitivity. Wang et al. found that baseline insulin sensitivity, as measured by clamp, accounted for nearly 40% of the variance in the clinical response to chromium.25 Subjects that were insulin resistant responded to chromium supplementation to a greater degree than those that were not insulin resistant.25
Table 3.
Intervention trials of chromium.
| Citation | Authors | N | Inclusion criteria | Effects of Chromium Supplementation |
|---|---|---|---|---|
| 7 | Anderson et al. | 17 | Hyperglycemia | Increased urinary chromium excretion. Lower OGTT glucose and glucagon levels compared to placebo. Lower insulin values compared to placebo. |
| 8 | Anderson et al. | 76 | Healthy individuals consuming a low-chromium diet for 14 weeks prior to supplementation. | No significant effects on FPG or or insulin levels from full sample. Chromium supplementation significantly decreased 90-min glucose and FPG levels of subjects with previously high glucose concentrations. Significant increase in FPG in subjects with previously low glucose concentrations. No changes in normoglycemic individuals. Serum insulin, weight, and lipids did not change. |
| 20 | Yazaki et al. | 80 | Overweight with abdominal adiposity | No change in BMI, body fat, FPG, or lipids. |
| 24 | Martin et al. | 25 | Type 2 diabetics using a sulfonylurea | Chromium significantly improved FPG, A1C, insulin sensitivity, and free fatty acid levels compared to placebo. Subjects randomized to placebo had increased weight, fat percent, total abdominal, visceral, and abdominal subcutaneous fat. |
| 25 | Wang et al. | 73 | Type 2 diabetes | Significant increase in insulin sensitivity compared to placebo. No effects on weight, body fat, BMI, fasting and AUC glucose and insulin. |
| 22 | Gunton et al. | 40 | Impaired glucose tolerance (IGT) | No difference in rate to progression or digression of IGT, glucose tolerance, FPG fasting insulin, HOMA-IR, and lipids. |
| 21 | Iqbal et al. | 63 | Abdominal obesity and metabolic syndrome | No effect on weight or waist circumference, lipids, insulin secretion, insulin sensitivity, FPG, vascular inflammation, or oxidative stress compared to placebo. No effects in persons with IGT. |
Strengths of our study include use of two doses commonly used in clinical practice and multiple outcome measures. Our study also incorporated a rigorous crossover design where subjects served as their own control, reducing variability in the results. A major limitation of the study is a lack of a biomarker assessing serum chromium levels at baseline and during therapy. Though current evidence suggests chromium deficiencies in humans are rare, it is possible that chromium deficient populations may respond to chromium supplementation. Other limitations include relatively broad inclusion criteria. It is recognized that patients with IFG and IGT, especially when those abnormalities are found in isolation (i.e. IFG alone or IGT alone) may represent different pathophysiological derangements and differential risks for developing future diabetes.26 Though subjects in our study were largely insulin resistant with a baseline mean HOMA-IR of 4.2, it is conceivable that a greater degree of insulin resistance may be required in order to detect a robust response to chromium. It is also possible that HOMA-IR, which as mentioned, is largely influenced by hepatic insulin sensitivity,26 is not refined enough a marker to exhibit an insulin sensitizing effect of chromium. Despite these concerns, if chromium were having any beneficial response on glucose metabolism in our subjects, improvement in some of the parameters we measured would be expected. We note, for example, that our post-challenge insulin levels (post-OGTT), which reflect both hepatic and peripheral insulin sensitivity,27 were unchanged as compared to the placebo group.
In conclusion, chromium supplementation does not appear to ameliorate insulin resistance or impaired glucose metabolism, and thus is unlikely to attenuate diabetes risk. Endocrinologists should therefore not endorse this therapy as part of a diabetes prevention strategy.
Acknowledgments
Technical assistance from Ryan McNally, Valentine Yanchou Njike, Moonsang Choi, Cynthia Kwong, Michelle Pinto-Evans, Julia O’Sullivan, and Tara Gaston is greatly appreciated. Nutritional supplements and placebo capsules used in this study were provided by Nutrition 21, Inc.
This study was supported by Grants Number R21 AT1332 and F32 AT002667 from the National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health (NIH) and grant U48 DP000053 from the Centers for Disease Control and Prevention (CDC). Insulin analysis took place at the Yale Center for Clinical Investigation Core Laboratory and was made possible by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the NIH, and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCCAM, CDC, NCRR, or NIH.
Biography

Ather Ali is Assistant Director of Integrative Medicine at the Yale-Griffin Prevention Research Center where he supervises Complementary and Alternative Medicine Research. He is co-director of the Integrative Medicine Center at Griffin Hospital and Associate Research Scientist in the Department of Pediatrics at the Yale School of Medicine. His research interests lie in chronic disease prevention, medically unexplained symptoms, and preventive medicine. He is one of the founders and on the leadership board of Integrative Medicine at Yale and is co-chair of the Research Working Group of the Consortium of Academic Health Centers for Integrative Medicine.
Footnotes
The authors have no relevant conflicts of interest.
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009 Jan;32(Suppl 1):S62–67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song Y, Manson JE, Tinker L, et al. Circulating levels of endothelial adhesion molecules and risk of diabetes in an ethnically diverse cohort of women. Diabetes. 2007 Jul;56(7):1898–1904. doi: 10.2337/db07-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padwal R, Majumdar SR, Johnson JA, Varney J, McAlister FA. A systematic review of drug therapy to delay or prevent type 2 diabetes. Diabetes Care. 2005 Mar;28(3):736–744. doi: 10.2337/diacare.28.3.736. [DOI] [PubMed] [Google Scholar]
- 4.Walker EA, Molitch M, Kramer MK, et al. Adherence to preventive medications: predictors and outcomes in the Diabetes Prevention Program. Diabetes Care. 2006 Sep;29(9):1997–2002. doi: 10.2337/dc06-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002 Feb 7;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallis M, Ruggiero L, Greene G, et al. Stages of change for healthy eating in diabetes: relation to demographic, eating-related, health care utilization, and psychosocial factors. Diabetes Care. 2003 May;26(5):1468–1474. doi: 10.2337/diacare.26.5.1468. [DOI] [PubMed] [Google Scholar]
- 7.Anderson RA, Polansky MM, Bryden NA, Canary JJ. Supplemental-chromium effects on glucose, insulin, glucagon, and urinary chromium losses in subjects consuming controlled low-chromium diets. Am J Clin Nutr. 1991 Nov;54(5):909–916. doi: 10.1093/ajcn/54.5.909. [DOI] [PubMed] [Google Scholar]
- 8.Anderson RA, Polansky MM, Bryden NA, Roginski EE, Mertz W, Glinsmann W. Chromium supplementation of human subjects: effects on glucose, insulin, and lipid variables. Metabolism. 1983 Sep;32(9):894–899. doi: 10.1016/0026-0495(83)90203-2. [DOI] [PubMed] [Google Scholar]
- 9.A scientific review: the role of chromium in insulin resistance. Diabetes Educ. 2004;(Suppl):2–14. [PubMed] [Google Scholar]
- 10.Federal Trade Commission. Companies advertising popular dietary supplement chromium picolinate can’t substantiate weight loss and health benefit claims, says FTC. [September 23, 2009];1996 http://www.ftc.gov/opa/1996/11/nut-21.shtm.
- 11.Dietary Supplement Fact Sheet: Chromium. Office of Dietary Supplements. National Institutes of Health; 2005. [5/6/2010]. http://ods.od.nih.gov/factsheets/chromium.asp. [Google Scholar]
- 12.NCEP Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002 Dec 17;106(25):3143–3421. [PubMed] [Google Scholar]
- 13.Biarnes J, Fernandez-Real JM, Fernandez-Castaner M, del Mar Garcia M, Soler J, Ricart W. Differential regulation of insulin action and tumor necrosis factor alpha system activity by metformin. Metabolism. 2005 Feb;54(2):235–239. doi: 10.1016/j.metabol.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000 Jan;23(1):57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 15.Ma Y, Njike VY, Millet J, et al. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: a randomized controlled crossover trial. Diabetes Care. Feb;33(2):227–232. doi: 10.2337/dc09-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faridi Z, Njike VY, Dutta S, Ali A, Katz DL. Acute dark chocolate and cocoa ingestion and endothelial function: a randomized controlled crossover trial. Am J Clin Nutr. 2008 Jul;88(1):58–63. doi: 10.1093/ajcn/88.1.58. [DOI] [PubMed] [Google Scholar]
- 17.Njike VY, Faridi Z, Shuval K, et al. Effects of sugar-sweetened and sugar-free cocoa on endothelial function in overweight adults. Int J Cardiol. 2009 Dec 23; doi: 10.1016/j.ijcard.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Katz DL, Nawaz H, Boukhalil J, et al. Effects of oat and wheat cereals on endothelial responses. Prev Med. 2001 Nov;33(5):476–484. doi: 10.1006/pmed.2001.0918. [DOI] [PubMed] [Google Scholar]
- 19.Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care. 2009 Nov;32(Suppl 2):S314–321. doi: 10.2337/dc09-S330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yazaki Y, Faridi Z, Ma Y, et al. A pilot study of chromium picolinate for weight loss. J Altern Complement Med. Mar;16(3):291–299. doi: 10.1089/acm.2009.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iqbal N, Cardillo S, Volger S, et al. Chromium picolinate does not improve key features of metabolic syndrome in obese nondiabetic adults. Metab Syndr Relat Disord. 2009 Summer;7(2):143–150. doi: 10.1089/met.2008.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunton JE, Cheung NW, Hitchman R, et al. Chromium supplementation does not improve glucose tolerance, insulin sensitivity, or lipid profile: a randomized, placebo-controlled, double-blind trial of supplementation in subjects with impaired glucose tolerance. Diabetes Care. 2005 Mar;28(3):712–713. doi: 10.2337/diacare.28.3.712. [DOI] [PubMed] [Google Scholar]
- 23.Broadhurst CL, Domenico R. Clinical studies on chromium picolinate supplementation in diabetes mellitus--a review. Diabetes Technol Ther. 2006 Dec;8(6):677–687. doi: 10.1089/dia.2006.8.677. [DOI] [PubMed] [Google Scholar]
- 24.Martin J, Wang ZQ, Zhang XH, et al. Chromium picolinate supplementation attenuates body weight gain and increases insulin sensitivity in subjects with type 2 diabetes. Diabetes Care. 2006 Aug;29(8):1826–1832. doi: 10.2337/dc06-0254. [DOI] [PubMed] [Google Scholar]
- 25.Wang ZQ, Qin J, Martin J, et al. Phenotype of subjects with type 2 diabetes mellitus may determine clinical response to chromium supplementation. Metabolism. 2007 Dec;56(12):1652–1655. doi: 10.1016/j.metabol.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faerch K, Borch-Johnsen K, Hoist JJ, Vaag A. Pathophysiology and aetiology of impaired fasting glycaemia and impaired glucose tolerance: does it matter for prevention and treatment of type 2 diabetes? Diabetologia. 2009 Sep;52(9):1714–1723. doi: 10.1007/s00125-009-1443-3. [DOI] [PubMed] [Google Scholar]
- 27.Man CD, Toffolo G, Basu R, Rizza RA, Cobelli C. Use of labeled oral minimal model to measure hepatic insulin sensitivity. Am J Physiol Endocrinol Metab. 2008 Nov;295(5):E1152–1159. doi: 10.1152/ajpendo.00486.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


