Abstract
Background
Chromoblastomycosis is a chronic fungal infection that affects skin and subcutaneous tissue. Lesions can be classified in tumorous, verrucous, cicatricial and plaque type. The cellular immune response in the severe form of the disease seems to correlate with a Th2 pattern of cytokines. The humoral immune response also seems to play a role. We intended to explore the populations of regulatory T cells and the Th17 pattern.
Methodology
Twenty-three biopsies of verrucous form were obtained from patients with clinical, culture and histopathological diagnostic of chromoblastomycosis, without treatment. It was performed an immunohistochemistry method to detect Foxp3, CD25, TGF-β, IL-6, IL-17 and IL-23.
Principal findings
IL-17 was the only cytokine with high expression in CBM when compared to normal skin. The expression of Treg cells, TGF- β, IL-6 and IL-23 were similar to normal skin.
Conclusions/Significance
The constitution of a local immune response with high expression of IL-17 and low expression of other cytokines could be at least in part, an attempt to help the immune system against fungal infection. On the other hand, high levels of local immune response mediated by Th17 profile could overcome the role of Treg cells. The inefficient immunomodulation as a consequence of the unbalance by Treg/Th17 cells seems to corroborate with the less effective immune response against fungi.
Author Summary
Chromoblastomycosis (CBM) is a chronic infection that affects skin and subcutaneous tissue, caused by some fungi which have a brownish color due to the presence of melanin pigments. The most frequent lesions are of verrucous type. Here we describe the participation of regulatory T cells and cells with the Th17 pattern of cytokines. High levels of Th17 cells participate in chronic inflammatory conditions, once at least in part they could improve the immune response and act in concomitance to the Th1 and Th2 patterns. Our results indicate a predominance of the Th17 pattern over Treg cells in verrucous lesions. We speculate that the local immune imbalance in CBM lesions characterized by exacerbated Th17 response, probably by suppressing the Treg response, is not effective for total fungal elimination. Even after long-period treatments, most patients has no absolute cure and often there is recurrence of the lesions. We believe that our study could contribute to the understanding of the immunopathogenesis of CBM and in such a way, presents some aspects to new possible therapies.
Introduction
Chromoblastomycosis (CBM) is a chronic granulomatous fungal infection that affects skin and subcutaneous tissue, especially the lower limbs. It is a cosmopolitan disease, but classically it is found in tropical and subtropical regions [1].
The causative agents of CBM are dimorphic fungi [2], [3] which have a brownish color due to the presence of melanin pigments in their cell wall and the spherical morphology that allow easy histological and mycological identification [4]–[8].
The infection occurs following traumatic inoculation of conidia or mycelial fragments from dematiaceous fungus [9]. The most frequently isolated species are Fonsecae pedrosoi, Phialophora verrucosa, Cladophialophora carrionii and eventually Rhinocladiella aquaspersa [10].
Clinically, the lesions can be classified in two instances: one that takes into account the appearance (tumorous, verrucous, cicatricial and plaque type) and the other, considering the gravity (mild, moderate or severe, according to the number and size of lesions) [11], [12], [13].
In general, CBM begins with the eruption of papules or nodules that develop slowly into warts. Regularly lesions appear in the lower limbs, knees and hands. There are reports of involvement of the face, chest, buttocks and other areas; dissemination via the lymphatic system is possible but infrequent [12].
The mechanisms of host defense in CBM are not fully elucidated. It is known that the immune response against the CBM is primarily cellular, where the process is ordered by phagocytic macrophages and humoral response also plays a role. Langerhans cells and Factor XIIIa+ dermal dendrocytes appear to be involved to a lesser extent in phagocytosis and antigen presentation against F. pedrosoi [6], [14]–[16].
Some studies have investigated the polarity of CBM and demonstrated, both experimentally and in situ, that the severe form of the disease, or warty lesions, correlates with a Th2 pattern of immune response by presenting the production of IL-10, high fungal burden, and also TNF-α. The average form is related to the Th1 profile, with high production of IFN-γ, low levels of IL-10, scarce number of fungi and relates to the better granulomatous immune response, resulting in less severe injuries (plaque type lesion) [17], [18].
Several studies have been conducted to better understand the Th1/Th2 paradigm of immune response or, in some diseases, the presence of both patterns of cytokines in the host. It is known that there is a subset of T lymphocytes that can modulate the immune response against pathogens, self-antigens and allergens which is also a constituent of immunological tolerance, called regulatory T cells (Treg). They are characterized by the expression of high levels of CD25 (α chain of the IL-2) whose function depends directly on the transcription factor Foxp3 [18]–[20].
A cytokine of high value for the studies of the immune response is TGF-β, since it is involved in the healing process in order to minimize tissue damage [21] and suppresses CD8+ cells [22], transform CD4+CD25− cells into CD4+CD25+ [23], induce naive T cells to differentiate into Foxp3+ [24] and still participates in differentiation of CD4 T cells in Th17 cells [25].
High concentrations of TGF-β added to the absence of pro-inflammatory cytokines direct the immune response to the development of regulatory T cells. Similarly, low concentrations of TGF-β associated with pro-inflammatory cytokines such as IL-1 β, IL-6, IL-21 and IL-23 promote expression of the IL-23 receptor (IL-23R), factor that allows the differentiation of CD4 T cells in Th17 [26], [27].
The Th17 lineage is a subpopulation of CD4+ T cells characterized by the secretion of IL-17 that seems to reinforce the protection of the host when the immune profiles of Th1 and Th2 cells are not totally effective against intracellular pathogens. Target of scientific spotlight, these cells appear to be protagonists in chronic inflammatory conditions, such as psoriasis [28], [29].
The study of both cell lines was performed by Pagliari et al. [30] in specimens from patients with paracoccidioidomycosis skin and mucous membranes lesions. The analysis revealed the involvement of both cellular profiles, identifying both the presence of immunoregulatory mechanism as the strengthening of effector T cells expressing IL-17.
Taking into account that, although the Th17 cells and regulatory T have similar ontogeny but distinct roles in the generation and control of infections [31], exploring their relationship could improve the understanding of the immunopathology of chromoblastomycosis and contribute to the most effective therapeutic strategies against the disease.
Materials and Methods
Biopsies
Twenty-three biopsies from skin lesions were kindly provided by Dermatopathology Laboratory, Division of Clinical Dermatology, Hospital das Clinicas, Faculty of Medicine, University of São Paulo, obtained from patients with clinical, culture and histological diagnostic of chromoblastomycosis by F. pedrosoi (86.96% males, mean age 61 years old, SD 15.28). The control group was constituted by ten specimens of normal skin, free of infectious or inflammatory activity at the time of surgery.
In addition to the skin control group without inflammatory activity (n = 10), it was also used a group of twenty skin lesions of paracoccidioidomycosis (PCM). This disease is caused by the dimorphic fungus Paracoccidioides brasiliensis and the host immune response against this fungus shares some similarities with chromoblastomycosis. Moreover, some markers of immune response proposed in this work have been described and discussed in PCM.
The use of the material that constituted the casuistic was approved by the ethics committee of Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, under the number 0317/11.
Immunohistochemistry
It was performed a streptavidin-biotin peroxidase method. The specimens were deparaffinized and hydrated in ethanol, the antigens were retrieved in TRIS/EDTA buffer pH 9.0 for 20 minutes at 95°C. The primary antibodies anti-Foxp3 (clone 236A/E7), anti-CD25 (clone 4C9), anti-TGF-β (clone TGFB17), IL-6 (clone 10C12), IL-17 (clone IL17A) and IL-23 (clone HLT2736) were diluted in 1% bovine albumin solution and incubated over-night at 4°C. Following, it was applied the second antibody and Streptavidin-peroxidase complex. 3,3-diaminobenzidine tetrahydroxychloride was used as chromogen and the slides were counterstained with hematoxylin. All reactions were performed with positive and negative controls. The second ones were constituted by the use of isotype controls and the omission of the primary antibody.
Quantitative analysis
Cells were quantified by counting the number of immunolabeled cells in nine randomized high-power fields for each specimen with an ×10 ocular lens with a square grid area of 0.0625 mm2. The number of positive cells was statistically analyzed with the Mann-Whitney test with the level of significance set at 95%.
Results
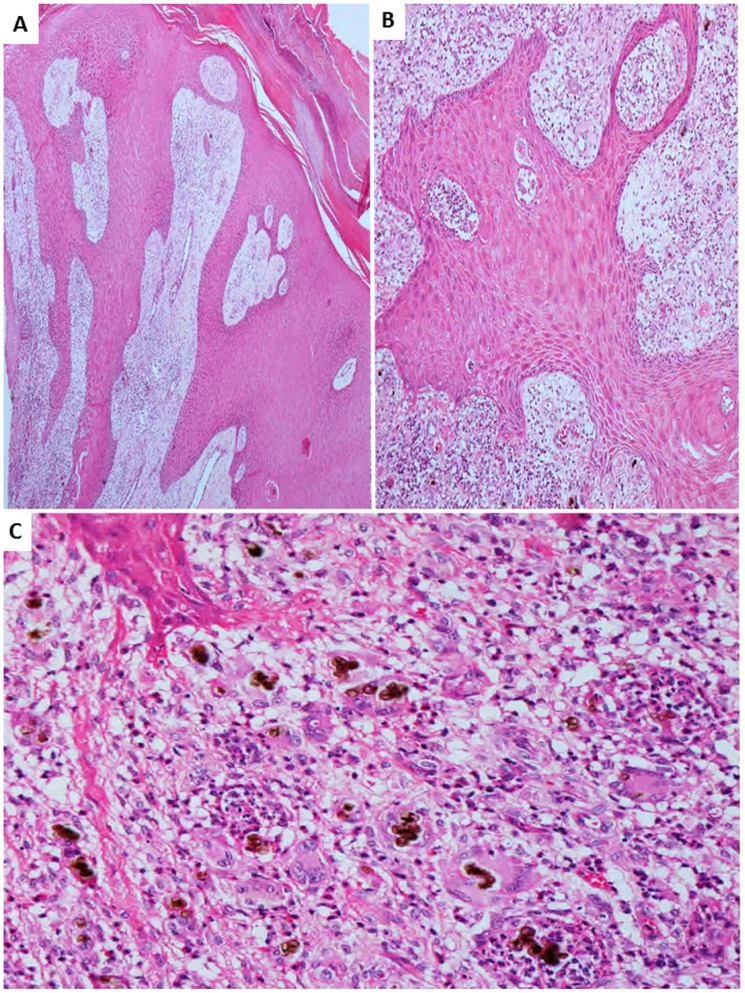
The group of CBM specimens consisted of lesions with a verrucous aspect. The histopathological analysis evidenced epidermal changes as hyperkeratosis, irregular acanthosis and microabscesses. The dermis was constituted by suppurative granulomas, inflammatory infiltrate consisting of giant cells, epithelioid cells, macrophages, lymphocytes, plasma cells, and eosinophils. The semi-quantitative analysis of parasitism ranged from moderate to intense. Eventually it was possible to identify ulceration of the skin, pseudocarcinomatous hyperplasia and fibrosis (Fig. 1).
Figure 1. Chromoblastomycosis – Histopathology of skin lesions.
A: epidermis presenting pseudocarcinomatous hyperplasia and acantosis (×40); B: presence of acantosis and intraepidermal microabscess (×100); C: dermal inflammatory infiltrate characterized by granulomatous response with giant cells, high number of fungal cells, eosinophils, macrophages and lymphocytes (×200) (hematoxylin-eosin).
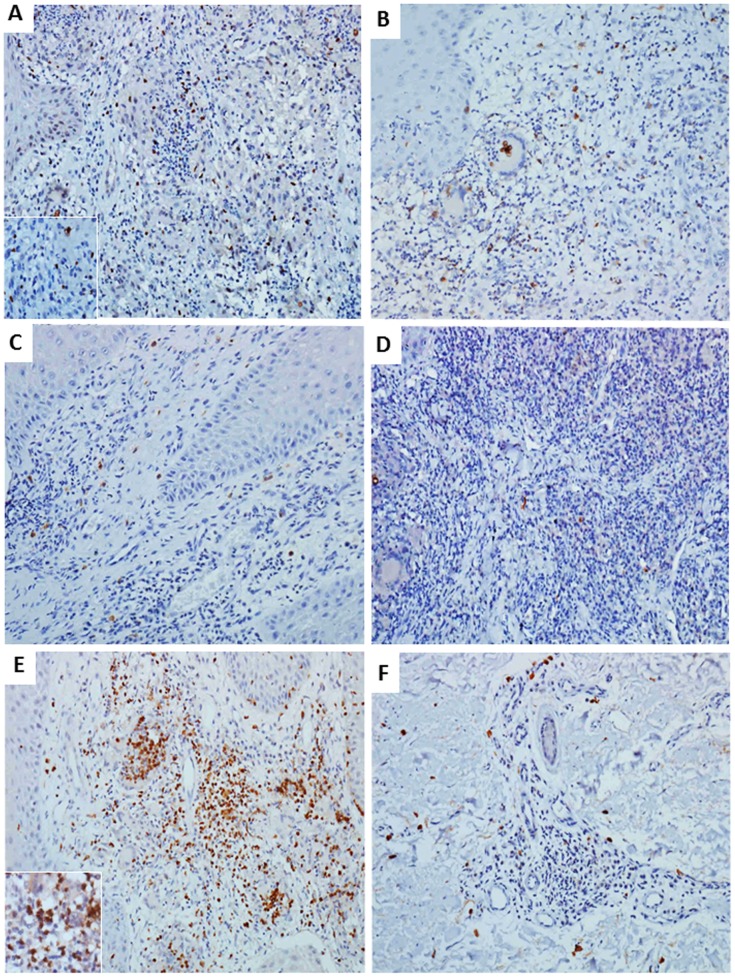
The immunohistochemical method allowed observing cells expressing Foxp3 and CD25, both in the inflammatory infiltrate and around granulomas. The expression of TGF-β was present in mononuclear cells of the inflammatory infiltrate. There was a discrete expression of IL-6. The expression of IL-17 was visualized in mononuclear and polymorphonuclear cells, mainly in granulomatous areas. Cells expressing IL-23 were present in the inflammatory infiltrates (Fig. 2 and 3).
Figure 2. Immunohistochemistry of representative chromoblastomycosis skin lesion.
A: Nuclear expression of Foxp3 in regulatory T cells. B: expression of CD25 in lymphocytes of the inflammatory infiltrate. C: Low number of cells expressing TGF-β. D: Scarce presence of IL-6. E: Intense number of T cells with expression of IL-17 in the inflammatory infiltrate. F: Low number of cells expressing IL-23 distributed in the lesion. Streptavidin-biotin peroxidase, immunostaining in brown (×200 and inset ×400).
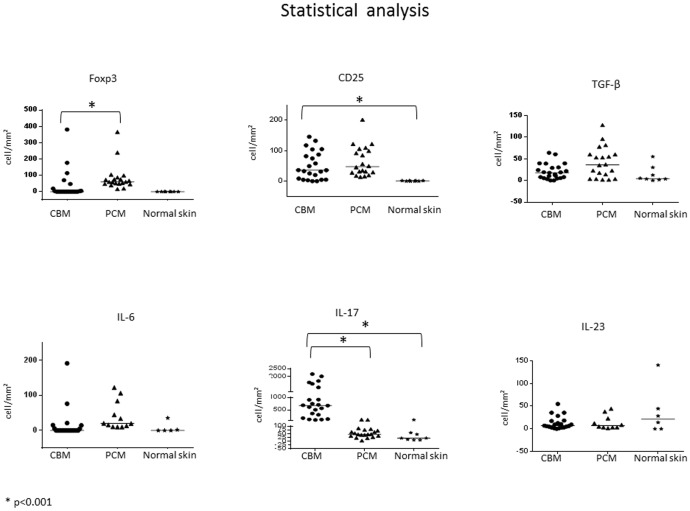
Figure 3. Comparative analysis among chromoblastomycosis, paracoccidioidomycosis and normal skin groups.
Distribution of number of cells/mm2 expressing Foxp3, CD25, TGF-β, IL-6, IL-23 and IL-17. Mann-Whitney test, * p<0.05.
The group of PCM was characterized by the presence of all markers, expressed in mononuclear cells in the inflammatory infiltrate similar to the CBM group.
The control group of normal skin also presented the markers. However, the expression was low or even absent in some cases.
Cells expressing IL-23 were detected in 66% of the normal skin specimens. Those cells were observed only in the dermis.
The statistical analysis of cells expressing Foxp3 evidenced a decreased number of such cells in CBM group when compared to PCM group (p<0.001) and similar number when compared to normal skin.
The expression of CD25 was similar between CBM and PCM groups, however CBM group presented a statistically significant higher number of positive cells when compared to normal skin (p<0.001).
The number of cells expressing TGF-β was similar among the three groups (p = 0.05), the same to IL-6 and IL-23 (p>0.05).
The three groups presented cells expressing IL-17. However, CBM group had an increased number when compared to the two other (p<0.001).
The quantitative analysis can be visualized in figure 3 and table S1.
Discussion
The study of CBM skin lesions is important because these injuries can reflect the immune status of the patient. The cutaneous tissue is often responsible for the initiation of immune cascade and, in the case of chromoblastomycosis, it is the structure to which the fungus has tropism.
Studies concerning immune response in CBM show that verrucous lesions present the mycotic granuloma formation with a pattern of Th2 response consistent with worse response against fungus. On the contrary, lesions of plaque type are characterized by a Th1 pattern of cytokines and therefore a better immune response, according to Minotto (unpublished data) and D'ávila [17]. However, there are no reports studying the immune dynamics of the lesions or inferences about what it takes to develop such responses.
We evidenced the predominance of cells expressing IL-17 and the presence of TGF- β and IL-23, although in low number. According to the literature published, this pattern of cytokines characterizes the Th17 immune response. The concomitant presence of a Th17 pattern could represent at first, an attempt of the in situ immune system to restrain the fungal infection.
With respect to the presence of regulatory T cells, although to a lesser extent when compared to cells expressing IL-17, we were also able to verify considerable number of Foxp3+ cells (40% of cases) and CD25+ cells (90%).
According to Melo and Carvalho [32], regulatory T cells have a key role in maintaining tolerance and regulation of the immune response. In the same way, in a work of Weaver [28] it is discussed that Th17 cells appear to be the translation of adaptability of the immune system favoring the protection of the host, so that the elucidation of the characteristics of both groups of cells, i.e. Treg and Th17 cells, could not only identify the mechanisms of invading organisms, but also to assist in the development of more effective therapies for numerous diseases.
Treg cells are characterized by a CD4+CD25+ phenotype [33], however the CD25 molecule is expressed by others populations of cells, such as others T cells and B lymphocytes or activated macrophages. Studies demonstrated that the gene Foxp3 has a nuclear expression in Treg cells and therefore a reliable marker of this cell line [34].
As already seen, the cytokine TGF-β plays a dichotomy role and mediates the targeting of Treg and Th17 responses. This selection between both profiles seems to depend on the levels of cells and the cytokines present in the microenvironment. High levels of TGF- β and low expression of pro-inflammatory cytokines favor the regulatory T-profile. On the contrary, low levels of TGF-β associated to significant presence of pro-inflammatory cytokines promote the synthesis of IL-23, development and maintenance of Th17 lymphocytes [35]–[41].
The cytokine IL-17 has received a considerable attention in many contexts since its discovery in 1993 [42]. The cytokine IL-23 promotes the secretion of IL-17 produced mainly by CD4+ T cells [43], [44].
According to many studies, high levels of Th17 cells are protagonists in chronic inflammatory conditions, once this cell population, at least in part, could improve the immune response and act in concomitance to the Th1 and Th2 patterns of response [28]. The performance of this cell line is characterized by secretion of IL-6, IL-17, IL-22 and TNF-α [45].
In the same context of investigation we consider patients affected by paracoccidioidomycosis (PCM), a systemic mycosis similar in some aspects to CBM. The disease follows inhalation of conidia of Paracoccidioides brasiliensis, a dimorphic fungus [46], and the primary focus are the lungs. It is described the cutaneous involvement at about 30% of cases. Unlike CBM, the lympho hematogenous spread of fungi is more frequent [47].
The cellular immune response in PCM is mainly mediated by macrophages and CD4+T cells [48], [49]. The Th1 profile of cytokines is considered the most important and some studies have also demonstrated the role of Treg cells and the profile of Th17 cytokines [30].
Considering previous investigations on the role of Treg cells in PCM, our results could suggest that this cell population not only have the capacity to interfere in the efficient immune response against fungi in chromoblastomycosis, but also benefit the host, by being able to reduce the tissue damage that follows a local immune response [30], [50].
In a recent study, the authors discussed the interaction of cells producing IL-17 and Treg cells and the homeostasis of the intestinal mucosal tissue [51]. The unregulated interaction of pro-inflammatory activity of IL-17 with pathogens seems to change the balance between regulatory and effector response predisposing the individual to the chronicity of the disease. It is noteworthy that even being subjected to long-period treatments, most patients affected by CBM has no absolute cure and often there is recurrence of the lesions. Thus, the unbalance between the populations of Treg/Th17 cells seems to restrain the effective immune response against the fungus.
Finally, it was interesting the similar number of cells expressing TGF-β, IL-6 and IL-23 when we compared the groups of lesions and normal skin. We expected that both CBM and PCM specimens presented more cells expressing those markers. We speculate that, at least in part, the presence of such cytokines in normal skin could be produced by the components of skin immune system [52].
Interestingly, in a previous work, Esterre et al. (1994) observed an overexpression of TGF-beta mainly at the periphery of the granulomas in areas of fibrosis [53].
In this work, we could suggest that the low number of cells with TGF-beta in lesions of CBM, with no difference from normal skin, could be explained by the randomized counting of positive cells throughout the dermis and not specifically in areas of fibrosis where such cells were also observed.
We suggest that our study could contribute to the understanding of the immunopathogenesis of chromoblastomycosis and in such a way, presents some aspects that could assist in the possibility of new therapies to modulate the immune system to the most effective immune profile of patients.
Supporting Information
Quantitative analysis: Numerical results of the markers studied. Results are given as mean ± standard deviation.
(TIF)
Funding Statement
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Brasil), grant number 2013/07994-1. AAdLS was supported by a master's degree fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Correia RTM, Criado PR, Valente NYS, Martins JEC (2010) Chromoblastomycosis:study of 27 cases and review of medical literature. An Bras Dermatol 85: 448–454. [DOI] [PubMed] [Google Scholar]
- 2. McGinnis MR (1983) Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol 8: 1–16. [DOI] [PubMed] [Google Scholar]
- 3. De Hoog GS, Queiroz-Telles F, Haase G, Fernandez-Zeppenfeldt G, Attili Angelis D, et al. (2000) Black fungi: clinical and pathogenic approaches. Med Mycol 38: 243–250. [PubMed] [Google Scholar]
- 4. Ellis DH, Griffiths DA (1974) The location and analysis of melanins in the cell walls of some soil fungi. Am J Microbiol 20: 1379–1428. [Google Scholar]
- 5. Ibrahim-Granet O, de Bievre C, Jendoubi M (1988) Immunochemical characterization of antigens and growth inhibition of Fonsecaea pedrosoi by species specific IgG. J Med Microbiol 26: 217–222. [DOI] [PubMed] [Google Scholar]
- 6. Alviano DS, Franzen AJ, Travassos LR, Holandino C, Rozental S, et al. (2004) Melanin from Fonsecaea pedrosoi induces production of human antifungal antibodies and enhances the antimicrobial efficacy of phagocytes. Infect Immun 72: 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Londero AT, Ramos CD (1976) Chromomycosis: a clinical and mycologic study of thirty-five cases observed in the hinterland of Rio Grande do Sul, Brazil. Am J Trop Med Hyg 25: 132–135. [DOI] [PubMed] [Google Scholar]
- 8. Torres-Guerrero E, Isa-Isa R, Isa M, Arenas R (2012) Chromoblastomycosis. Clinics in Dermat 30: 403–408. [DOI] [PubMed] [Google Scholar]
- 9. Carrion AL (1950) Chromoblastomycosis. Ann NY Acad Sci 50: 1255–1282. [DOI] [PubMed] [Google Scholar]
- 10. Silva JP, de Souza W, Rozental S (1998–1999) Chromoblastomycosis: a retrospective study of 325 cases on Amazonic Region (Brazil). Mycopathologia 143: 171–175. [DOI] [PubMed] [Google Scholar]
- 11. Zaror L, Fischman O, Pereira CA, Felipe RG, Gregório LC, et al. (1987) A case of primary nasal chromoblastomycosis. Mykosen 30: 468–471. [PubMed] [Google Scholar]
- 12. Queiroz-Telles F, McGinnis MR, Salkin I, Graybill JR (2003) Subcutaneous mycoses. Infect Dis Clin North Am 17: 59–85. [DOI] [PubMed] [Google Scholar]
- 13. Queiroz-Telles F, Esterre P, Perez-Blanco M, Vitale RG, Salgado CG, et al. (2009) Chromoblastomycosis: an overview of clinical manifestations, diagnosis and treatment. Med Mycol 47: 3–15. [DOI] [PubMed] [Google Scholar]
- 14. Farbiarz SR, Carvalho TU, Alviano C, Souza W (1992) Inhibitory effect of melanin on the interaction of Fonsecaea pedrosoi with mammalian cells in vitro. J Med Vet Mycol 30: 265–273. [PubMed] [Google Scholar]
- 15. Sotto MN, De Brito T, Silva AM, Vidal M, Castro LG (2004) Antigen distribution and antigen-presenting cells in skin biopsies of human chromoblastomycosis. J Cutan Pathol 31: 14–18. [DOI] [PubMed] [Google Scholar]
- 16. Silva JP, Silva MB, Salgado UI, Diniz JAP, Rozental S, et al. (2007) Phagocytosis of Fonsecaea pedrosoi conidia, but not sclerotic cells caused by Langerhans cells, inhibitsCD40 and B7-2 expression. FEMS Immunol Med Microbiol 50: 104–111. [DOI] [PubMed] [Google Scholar]
- 17. D'Avila SC, Pagliari C, Duarte MI (2003) The cell-mediated immune reactions in cutaneous leisons of chromoblastomycosis and their correlation with different clinical forms of the disease. Mycopathologia 156: 51–60. [DOI] [PubMed] [Google Scholar]
- 18. Mazo Fávero Gimenes V, Da Gloria DM, Ferreira KS, Marques SG, Goncalves AG, et al. (2005) Cytokines and lymphocyte proliferation in patients with different clinical forms of Chromoblastomycosis. Microbes Infect 7: 708–713. [DOI] [PubMed] [Google Scholar]
- 19. Campbell DJ, Ziegler SF (2007) Foxp3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol 7: 305–310. [DOI] [PubMed] [Google Scholar]
- 20. Sojka DK, Huang YH, Fowell DJ (2008) Mechanisms of regulatory Tcell suppression - a diverse arsenal for a moving target. J Immunology 124: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parise-Fortes MR, Miot HÁ, Kurokawa CS, Marques MEA, Marques AS (2011) Imunologia da paracoccidioidomicose. An Bras Dermatol 86: 516–525.21738969 [Google Scholar]
- 22. Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, et al. (2005) Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo . Proc Natl Acad Sci 102: 419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pyzick M, Piccirillo CA (2007) TGF-beta1 modulates Foxp3 expression and regulatory activity in distinct CD4+ T cell subsets. J Leuckoc Biol 82: 335–346. [DOI] [PubMed] [Google Scholar]
- 24. Chen W, Jin W, Hardegen N, Lei KJ, Li L, et al. (2003) Conversion of peripheral CD4+CD25− naïve T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 198: 1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, et al. (2006) Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441: 231–234. [DOI] [PubMed] [Google Scholar]
- 26. Manel N, Unutmaz D, Littman DR (2008) The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol 9: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang Y, Xu J, Niu Y, Bromberg JS, Ding Y (2008) T-bet and eomesodermin play critical roles in directing T cell differentiation to Th1 versus Th17. J Immunol 181: 8700–8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weaver CT, Hatton RD, Mangan PR, Harrington LE (2007) IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 25: 821–852. [DOI] [PubMed] [Google Scholar]
- 29. Di Cesare A, Di Meglio P, Nestle FO (2009) The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Inv Dermatology 129 (2009) 1339–1350. [DOI] [PubMed] [Google Scholar]
- 30. Pagliari C, Fernandes ER, Stegun FW, da Silva WL, Seixas Duarte MI, et al. (2011) Paracoccidioidomycosis: cells expressing IL17 and Foxp3 in cutaneous and mucosal lesions. Microb Pathog 50: 263–267. [DOI] [PubMed] [Google Scholar]
- 31. Basso AS, Cheroutre H, Mucida D (2009) More stories on Th17 cells. Cell Res 19: 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Melo KM, Carvalho BTC (2009) Células T regulatórias: mecanismos de ação e função nas doenças humanas. Rev Bras Alerg Imunopatol 32: 184–188. [Google Scholar]
- 33. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M (1995) Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmunediseases. J Immunol 155: 1151–1164. [PubMed] [Google Scholar]
- 34. Fontenot JD, Gavin MA, Rudensky AY (2003) Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunology 4: 330–336. [DOI] [PubMed] [Google Scholar]
- 35. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, et al. (2006) Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature 441: 235–238. [DOI] [PubMed] [Google Scholar]
- 36. Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suárez-Fariñas M, et al. (2007) Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med 204: 3183–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. da Silva JP, da Silva MB, Campelo SR, Salgado UI, Diniz JA, et al. (2010) TGF-beta plasma levels in chromoblastomycosis patients during itraconazole treatment. Cytokine 51: 202–6. [DOI] [PubMed] [Google Scholar]
- 38. Palm NW, Medzhitov R (2007) Antifungal defense turns 17. Nat Immunol 8: 549–51. [DOI] [PubMed] [Google Scholar]
- 39. Quaresma JA, de Oliveira E, Cardoso de Brito A (2008) Is TGF-beta important for the evolution of subcutaneuos chronic mycoses? Med Hypotheses 70: 1182–5. [DOI] [PubMed] [Google Scholar]
- 40. Romani L (2011) Immunity to fungal infections. Nat Rev Immunol 11: 275–88. [DOI] [PubMed] [Google Scholar]
- 41. Guha M, Xu ZG, Tung D, Lanting L, Natarajan R (2007) Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J 21: 3355–68. [DOI] [PubMed] [Google Scholar]
- 42. Rouvier E, Luciani MF, Mattéi MG, Denizot F, Golstein P (1993) CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol 150: 5445–5456. [PubMed] [Google Scholar]
- 43. Manel N, Unutmaz D, Littman DR (2008) The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol 9: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang Y, Xu J, Niu Y, Bromberg JS, Ding Y (2008) T-bet and eomesodermin play critical roles in directing T cell differentiation to Th1 versus Th17. J Immunol 181: 8700–8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McKenzie BS, Kastelein RA, Cua DJ (2006) Understanding the IL-23-IL-17 immune pathway. Trends Immunol 27: 17–23. [DOI] [PubMed] [Google Scholar]
- 46. Carbonell LM (1967) Cell wall changes during the budding process of paracoccidioides brasiliensis and blastomyces dermatitidis. J Bacteriology 94: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Franco M, Peracoli MT, Soares A, Montenegro R, Mendes RP, et al. (1993) Host-parasite relationship in Paracoccidioidomycosis. Curr Top Med Mycol 5: 115–149. [PubMed] [Google Scholar]
- 48. Cano LE, Kashino SS, Arruda C, André D, Xidieh CF, et al. (1998) Protective role of gamma interferon in experimental pulmonary paracoccidioidomycosis. Infect Immunol 66: 800–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pagliari C, Sotto MN (2003) Dendritic cells and pattern of cytokines in paracoccidioidomycosis skin lesions. Am J Dermatopathol 2003 25: 107–12. [DOI] [PubMed] [Google Scholar]
- 50. Cavassani KA, Campanelli AP, Moreira AP, Vancim JO, Vitali LH, et al. (2006) Systemic and local characterization of regulatory T cells in a chronic fungal infection in humans. J Immunol 177: 5811–5818. [DOI] [PubMed] [Google Scholar]
- 51. Littman DR, Rudensky AY (2010) Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140: 845–858. [DOI] [PubMed] [Google Scholar]
- 52. Bos JD, Kapsenberg ML (1993) The skin immune system progress in cutaneous biology. Immunol Today 14: 75–8. [DOI] [PubMed] [Google Scholar]
- 53. Esterre P, Lortat-Jacob H, Sainte-Marie D, Pradinaud R, Grimaud JA (1994) The potential role of cytokines in the immunopathology of chromomycosis. J Mycol Med 4: 145–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quantitative analysis: Numerical results of the markers studied. Results are given as mean ± standard deviation.
(TIF)