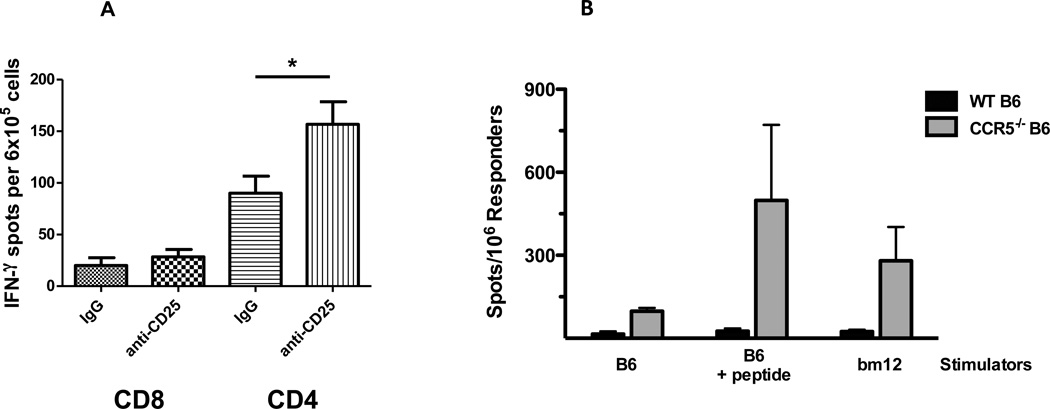
Figure 9.
Absence of bm12-reactive CD8 T cell priming in wild type C57BL/6 recipients of bm12 heart allografts. (A) Groups of C57BL/6 mice received B6.H-2bm12 heart allografts and were treated with 250 µg anti-CD25 mAb on days −1, +1, +3, +5, +7 and +9 post-transplant. On day 18 post-transplant, CD4 and CD8 T cells were purified from each recipient spleen and the frequency of bm12 donor-reactive CD4 and CD8 T cells producing IFN-γ was quantified by ELISPOT assay. The results indicate the mean number of spots for the splenic T cell populations from 4 individual mice per group ± SEM. *p < 0.05. (B) Groups of wild type C57Bl/6 and B6.CCR5-deficient mice were immunized subcutaneously with a mixture of 2 Kb- plus 2 Db-restricted I-Abm12 β chain peptides emulsified in Complete Freund’s Adjuvant and 12 days later the CD8 T cells were purified from each of the spleens. The frequency of CD8 T cells producing IFN-γ during culture with syngeneic C57Bl/6 or bm12 stimulator cells was quantified by ELISPOT assay. In the indicated cultures, 20 µg of the I-Abm12 β chain peptides were added at the initiation of the ELISPOT cultures with the C57Bl/6 stimulator cells. The results indicate the mean number of spots for splenic CD8 T cells from 4 individual mice per group ± SEM. *p < 0.05.