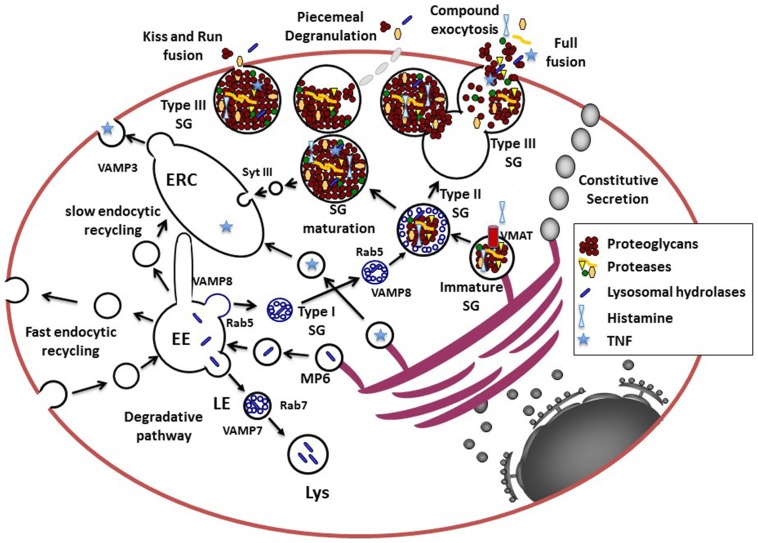
Figure 1.
Model for the secretory pathways in MC. Newly synthesized proteins destined for secretion enter the secretory pathway at the rough ER. After passage through the Golgi where posttranslational modifications occur, they reach the trans-Golgi network (TGN), which functions as a sorting hub depending on various structural motifs. For example, GPI-anchored or N-and O-glycosylated proteins enter the constitutive secretory pathway for trafficking to the cell surface. Proteins destined for the endosomal–lysosomal pathway are modified by Mannose-6 phosphate (MP6) for recognition by the Mannose-6-phosphate receptor that via an acidic cluster/dileucine motif is delivered to the early endosome (EE). Together with endocytosed proteins destined for degradation they are further sorted to late endosomes (LE) forming multivesicular structures and then fuse with lysosomes (Lys) via a VAMP-7 and Rab7-dependent pathway. Some proteins, like for example, newly synthesized TNF may be sorted into vesicles to reach the endocytic recycling compartment (ERC) from where it could be secreted. The ERC may serve as an additional sorting hub for the exocytosis and recycling of proteins via retrograde pathways (not shown in this figure). The cytoplasmic SG, which contain proteins destined for the regulated secretory pathway for release upon stimulation in MC and other hematopoietic cells are so-called mixed type organelles or secretory lysosomes carrying features of endosomes and lysosomes. Proteins destined for SG may leave the TGN at sites where larger protein aggregates are formed possibly via association to highly negatively charged proteoglycans. The immature SG formed may then rapidly fuse with carriers (type I SG) containing small intraluminal vesicles derived from the early endosome via a Rab5 and VAMP-8 dependent mechanism to form type II SG. Some inflammatory mediators like histamine incorporates into SG via specific vesicular monoamine transporters (VMAT). SG then undergo a maturation process, which generates mature type III SG by the retrieving cargo of missorted proteins and intraluminal vesicles to the ERC. Pre-formed inflammatory mediators can then be released via several types of fusion processes including transient kiss-and-run fusion, piecemeal degranulation, or multigranular/compound exocytosis, which involves full fusion and collapse of SG. It is possible that during stimulation all types of granules (type I, II, and III) are fusion competent as suggested by the fact that cultured cells with less mature granules can be stimulated for release.