Abstract
Objective
To compare gadoxetic acid injection rates of 0.5 mL/s and 1 mL/s for hepatic arterial-phase magnetic resonance (MR) imaging.
Materials and Methods
In this prospective study, 101 consecutive patients with suspected focal liver lesions were included and randomly divided into two groups. Each group underwent dynamic liver MR imaging using a 3.0-T scanner after an intravenous injection of gadoxetic acid at rates of either 0.5 mL/s (n = 50) or 1 mL/s (n = 51). Arterial phase images were analyzed after blinding the injection rates. The signal-to-noise ratios (SNRs) of the liver, aorta, portal vein, hepatic vein, spleen, and pancreas were measured. The contrast-to-noise ratios (CNRs) of the hepatocellular carcinomas (HCC) were calculated. Finally, two experienced radiologists were independently asked to identify, if any, HCCs in the liver on the images and score the image quality in terms of the presence of artifacts and the proper enhancement of the liver, aorta, portal vein, hepatic vein, hepatic artery, spleen, pancreas, and kidney.
Results
The SNRs were not significantly different between the groups (p = 0.233-0.965). The CNRs of the HCCs were not significantly different (p = 0.597). The sensitivity for HCC detection and the image quality scores were not significantly different between the two injection rates (p = 0.082-1.000).
Conclusion
Image quality and sensitivity for hepatic HCCs of arterial-phase gadoxetic acid-enhanced MR were not significantly improved by reducing the contrast injection rate to 0.5 mL/s compared with 1 mL/s.
Keywords: Gadoxetic acid, Injection rate, Hepatocellular carcinoma, Magnetic resonance imaging
INTRODUCTION
Gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (hereafter, gadoxetic acid; Primovist, Bayer Healthcare, Berlin, Germany) is an approved contrast agent for dynamic liver magnetic resonance (MR) imaging (1, 2, 3, 4, 5, 6, 7, 8, 9). Gadoxetic acid-enhanced MR imaging allows for improved detection and characterization of focal hepatic lesions, when compared to both contrast-enhanced computed tomography (CT) and gadolinium-diethylenetriamine penta-acetic acid (Gd-DTPA)-enhanced MR imaging, because of the highly specific uptake of the contrast agent by hepatocytes (10, 11, 12).
Clinically, the use of gadoxetic acid ensures good contrast between normal hepatocytes and lesions without functioning hepatocytes in hepatobiliary imaging (8, 13). However, adequate quality of arterial-phase images is also crucial to the detection and characterization of hypervascular focal hepatic lesions. As gadoxetic acid has higher relaxivity in human plasma than Gd-DTPA, improved image quality with a lower dose of gadoxetic acid is possible. The recommended dosage of gadoxetic acid is 0.025 mmol/kg of body weight (i.e., 0.1 mL/kg of body weight), which is one-fourth of the recommended dosage of Gd-DTPA (0.1 mmol/kg of body weight) (14, 15, 16, 17). At many institutions, an injection rate of 1 or 2 mL/s is conventionally used for gadoxetic acid-enhanced liver MR imaging.
The total injection amount of gadoxetic acid is 6-10 mL in patients weighing 60-100 kg. In such patients, the total injection durations are approximately 6-10 seconds and 3-5 seconds at injection rates of 1 and 2 mL/s, respectively, and hepatic arterial-phase MR imaging usually takes approximately 20 seconds.
Therefore, proper acquisition of hepatic arterial-phase images with gadoxetic acid may be difficult without using a bolus-tracking method or test-bolus injection technique. In one study, gadoxetic acid injection rates of 1 and 2 mL/s ensured comparable image quality and detection of focal hypervascular hepatic lesions on a 3-T MR system (18). On the other hand, another study showed that a 1 mL/s injection of gadoxetic acid achieved greater enhancement of the aorta and aortic perfusion parameters than 2 mL/s on a 1.5-T MR system (19). Moreover, an animal study showed a significant improvement in the arterial enhancement of the aorta when the injection rate of gadoxetic acid was reduced from 2 mL/s to 1 mL/s (20).
By using a lower injection rate, the bolus is stretched, not compacted, and has a greater chance to establish the protein binding process in human blood, which results in a higher relaxivity that can avoid saturation effects (21). A slower rate of 1 mL/s may be useful to prolong the injection duration, given the lower volume. Thus, we expect that using a lower injection rate can achieve improvements in arterial enhancement and decrease the patient's subjective discomfort from the intravenous administration route. In patients weighing 60-100 kg, total injection durations are approximately 12-20 seconds at injection rate of 0.5 mL/s, which is similar to usual hepatic arterial-phase MR imaging acquisition time.
To our knowledge, however, there has been no study with a prospective design focusing on an evaluation of the effect of a gadoxetic acid injection rate slower than 1 mL/s. Therefore, using a randomized study, we intended to compare gadoxetic acid injection rates of 0.5 mL/s and 1 mL/s for hepatic arterial-phase MR imaging with regard to image quality and detection of hepatocellular carcinomas (HCCs).
MATERIALS AND METHODS
Design
In this empirical randomized study, gadoxetic acid injection rate of 0.5 mL/s was compared to that of 1 mL/s for hepatic arterial-phase MR imaging. Given the empirical nature of the study, sample size calculation was not performed and we decided to include at least 50 patients per group, based on previous similar studies (18, 19). Randomization was performed based on a random number generated by commercially available software (SPSS for Windows release 20.0; IBM, Inc., Armonk, NY, USA).
Subjects
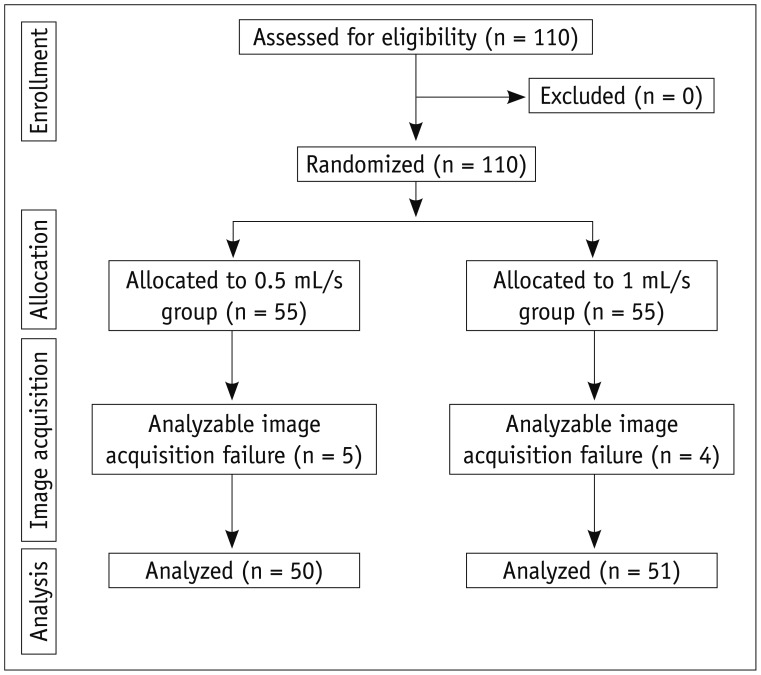
This prospective study was approved by the Institutional Review Board at our institute. A written informed consent was obtained from each patient. Between October and November 2011, 110 patients who were scheduled to undergo dynamic liver MR imaging for the suspicion of focal liver lesions according to the findings on ultrasound, CT, or laboratory tests were eligible to be included in this study. The exclusion criteria of this study were as follows: 1) allergy to gadolinium-based contrast agents, 2) impaired renal function, and 3) a contraindication to MR imaging (patients with electrical or magnetic implants or claustrophobia). We identified 110 consecutive eligible patients. All patients agreed to participate and were randomized into two groups (Fig. 1). The patients who were scheduled to undergo dynamic liver MR imaging using gadoxetic acid at an injection rate of 0.5 mL/s were assigned to 0.5 mL/s group (n = 55), and the patients who were scheduled to undergo with an injection rate of 1 mL/s were assigned to 1 mL/s group (n = 55).
Fig. 1.
Consolidated standards of reporting trials flow diagram.
The presence of chronic liver disease was determined according to the medical record including the presence of alcoholic liver disease, viral marker positive of hepatitis B virus, or hepatitis C virus, and the previous radiological examinations were reviewed by a radiologist who was blinded to the injection rates. The presence of HCCs, which was to be used as the reference standard for analysis of diagnostic sensitivity, was determined according to the pathological results including percutaneous biopsy (n = 4) and surgery (n = 5) or characteristic imaging findings (n = 33). The characteristics of a HCC on MR imaging were defined by early enhancement on hepatic arterial phase images and washout on the late phases of contrast-enhanced MR images (22, 23, 24). Among 33 patients diagnosed with HCC by characteristic imaging findings, 16 patients underwent transarterial chemoembolization and showed lipiodol uptake in follow-up imaging study, 4 patients showed marked size increment of mass in follow-up imaging study, and 13 patients underwent radiofrequency ablation.
MRI Technique
Magnetic resonance examinations were performed with a 3.0-T whole-body MR system (Magnetom Tim Trio, Siemens AG, Munich, Germany) using an 8-channel phased-array coil. The MR imaging protocols were as follows. For contrast-enhanced MR imaging, the unenhanced images were obtained using fat-suppressed T1-weighted gradient-echo in-phase imaging (repetition time [TR]/echo time [TE], 3.4/1.3 ms; flip angle, 13°; matrix, 320 × 176; bandwidth, 500 Hz per pixel; Grappa factor, 2) with a 3.5 mm section thickness, no intersection gap, and a field-of-view of 360 × 260 mm. A dosage of 0.1 mL/kg (0.025 mmol/kg gadoxetic acid) contrast agent was injected intravenously as a bolus at a rate of 0.5 mL/s or 1 mL/s followed by a 20 mL saline chaser at the same injection rate as the contrast agent.
Arterial-phase imaging was obtained using combined applications to reduce exposure (CARE, Siemens Healthcare, Erlangen, Germany) bolus technique. Dynamic phase (60, 100, and 180 seconds after contrast injection) and hepatobiliary phase (10 and 20 minutes after contrast injection) images were obtained using fat-suppressed T1-weighted gradient-echo in-phase imaging with a 3.5 mm section thickness, no intersection gap, and a field-of-view of 360 × 260 mm.
Quantitative Image Analysis
The mean signal intensities (SIs) were measured in the right and left lobes of the liver, spleen, pancreas, abdominal aorta, hepatic vein, and portal vein, using an approximately 20 mm diameter circular area, and the background noise was measured in an approximately 50 mm diameter circular area. Large vessels, dilated biliary ducts, prominent artifacts, and suspicious tumors were avoided in the hepatic, splenic, and pancreatic measurements.
The SI of the liver was defined as the mean of the signal intensities of the right and left lobes. The SIs of the hepatic and portal veins were measured in the proximal portion of the right hepatic vein and the confluence level of the main portal vein, respectively. The SIs of tumors were measured in a circular area with a diameter that covered most of the enhancing portion of the tumor, while avoiding regions of cystic or necrotic change. If multiple tumors were found, the SI of the largest tumor was measured. The SIs were measured by a radiologist (two years of experience in abdominal imaging) who was blinded to the injection rates and clinical information.
The signal-to-noise ratios (SNRs) for the liver, spleen, pancreas, abdominal aorta, hepatic vein, and portal vein were calculated by dividing the SI of each organ by one standard deviation (SD) of the background SI (SNR = SI of the organ / SD of background SI). The contrast-to-noise ratio (CNR) for hepatic tumors was calculated by dividing the difference between the SI of the tumor by that of liver with one SD of the background SI (CNR = [SI of the tumor - SI of the liver] / SD of background SI).
Sensitivity for HCC Detection
Two radiologists (with six and eight years of experience in abdominal imaging and a fellowship in abdominal radiology, respectively), blinded to the injection rates and clinical information, independently reviewed only the arterial phase images. They recorded the number, size, and location of intrahepatic HCCs detected on hepatic arterial-phase images. To diagnosis HCC, the entire phase of MR imaging was used by radiologists who did not review hepatic arterial-phase images.
Qualitative Imaging Analysis
To evaluate the image quality of the hepatic arterial phase, the same radiologists reviewed hepatic arterial-phase images while using unenhanced images as a reference. The following nine items were used in the qualitative analysis of the hepatic arterial phase images: artifacts, good abdominal-aortic enhancement, strong hepatic-arterial enhancement, partial portal-venous enhancement, absence of hepatic-venous enhancement, minimal enhancement of the liver, zebra effect of the spleen, good pancreatic enhancement, and good renal corticomedullary differentiation. For respiratory artifacts, a severe artifact was scored as 1, a mild artifact was scored as 2, and no artifact was scored as 3. Other items were scored as 1 when the image was not satisfactory and as 2 when the image was satisfactory (18). To determine the overall image quality, the scores of all items were summed. A higher score represented better image quality.
Statistical Analysis
All statistical analyses were performed with commercially available software (SPSS for Windows release 20.0; IBM, Inc.). An unpaired t test was used to analyze differences in mean body weight, age, diameter of the HCC, SNR, and CNR between the groups. A Mantel-Haenszel chi-squared test or Fisher's exact test was used to compare the proportion of patients with chronic liver disease, proportion of patients with HCC, proportion of patients with main portal vein thrombosis, sensitivity for HCC detection on hepatic arterial-phase images between the examiners and between the two different injection rates, and proportion of analyzable image acquisition failure between the groups. Values of p < 0.05 were considered as statistically significant.
RESULTS
Subjects
Of the 55 patients assigned to 0.5 mL/s group, five patients were excluded because of analyzable image acquisition failure, and 50 patients were analyzed (Fig. 1). In 1 mL/s group, four patients were excluded because of analyzable image acquisition failure, and 51 patients were analyzed (Fig. 1). All cases of analyzable image acquisition failure were caused by severe respiratory motion artifact due to poor patient cooperation during MR imaging. Table 1 shows the characteristics of 101 patients who were finally included. There were 66 men and 35 women, and their mean (range) age was 59 (28-85) years. Fifty-seven patients had chronic liver disease. The mean (range) patient age was 60.3 (35-81) years in the 0.5 mL/s group and 57.0 (28-85) years in the 1 mL/s group. The mean (range) body weight was 66.5 (43-86) and 61.1 (45-93) kg in the 0.5 and 1 mL/s groups, respectively. No significant difference in age (p = 0.251) or body weight (p = 0.136) was noted between the groups. Analyzable image acquisition failure occurred in 5 cases in the 0.5 mL/s group and in 4 cases in the 1 mL/s group. No significant difference in the proportion of analyzable image acquisition failure (p = 1.000) was noted between the two groups.
Table 1.
Characteristics of Study Groups
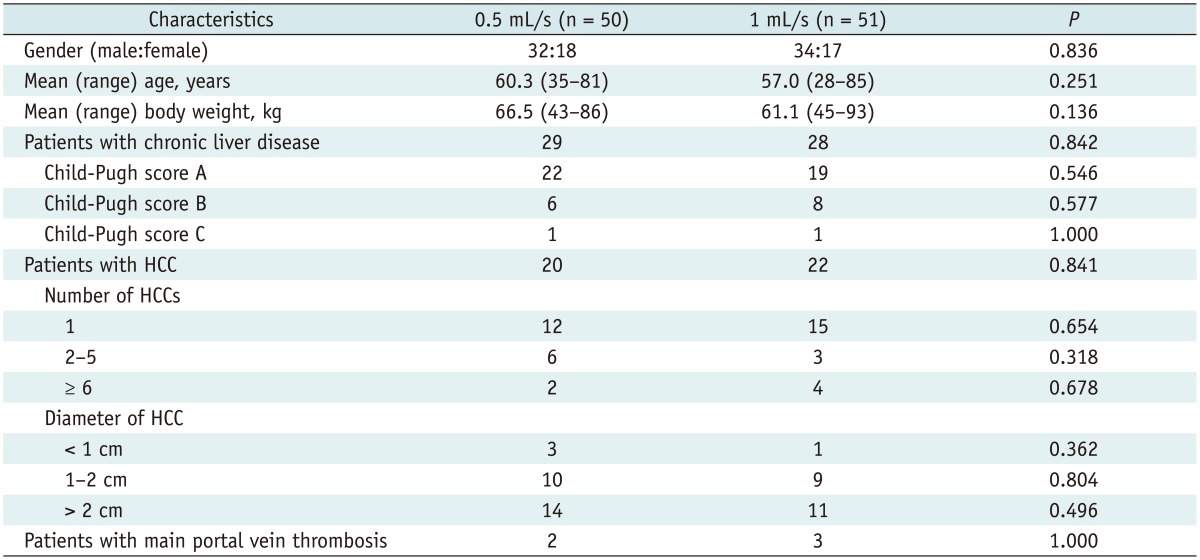
Note.-HCC = hepatocellular carcinoma
Quantitative Analysis
The mean (range) scan delay after aortic enhancement was 8.4 (7-12) seconds in the 0.5 mL/s group and 8.6 (7-13) seconds in the 1 mL/s group. The mean (range) arterial image acquisition time was 14.3 (13-15) seconds in the 0.5 mL group and 14.1 (13-15) seconds in the 1 mL/s group. The actual contrast injection time was 4.5-9 seconds in the 0.5 mL group and 9-17 seconds in the 1 mL/s group.
Table 2 presents the SNRs of each organ and the tumor-to-liver CNRs from each group. The SNRs of the liver, aorta, portal vein, spleen, and pancreas in the 0.5 mL/s group were higher than those of the 1 mL/s group. However, the SNRs of the liver, portal vein, hepatic vein, spleen, and pancreas were not significantly different between the two groups (p = 0.233-0.965). Similarly, the tumor-to-liver CNRs of the HCCs in the 1 mL/s group were higher than those of the 0.5 mL/s group, and the CNRs of HCCs were not significantly different between the two groups (Figs. 2, 3).
Table 2.
Results of Quantitative Analysis of Hepatic Arterial-Phase Images Obtained Using Different Injection Rates of Gadoxetic Acid
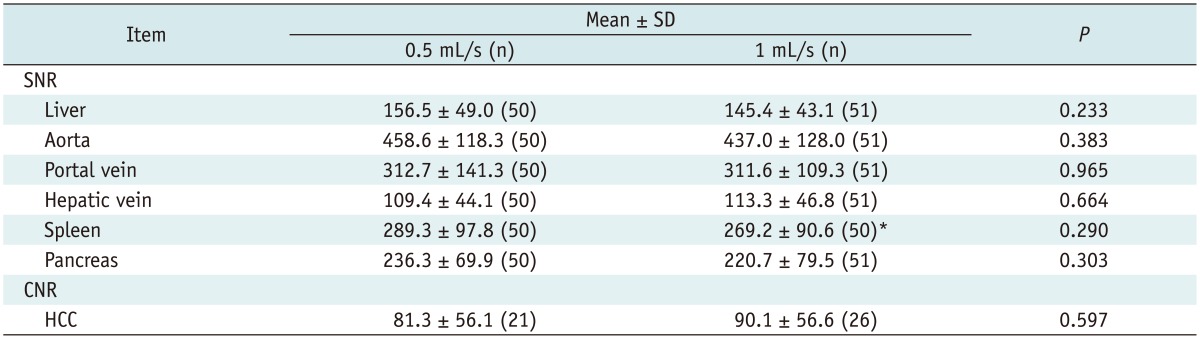
Note.-*Splenectomy state. CNR = contrast-to-noise ratio, HCC = hepatocellular carcinoma, SD = standard deviation, SNR = signal-to-noise ratio
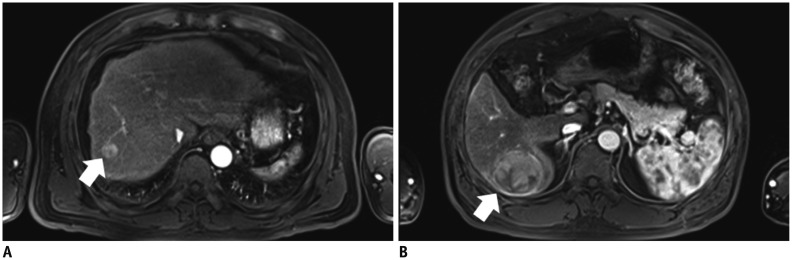
Fig. 2.
Arterial phase MR images obtained after intravenous injection of gadoxetic acid at rate of 1 mL/s.
A, B. 55-year-old man with hepatocellular carcinoma (HCC) and liver cirrhosis. A. Arterial-phase three-dimensional gradient-echo (repetition time/echo time = 3.4/1.3 ms; flip angle = 13°) MR images obtained after intravenous injection of gadoxetic acid at rate of 0.5 mL/s. Strong enhancement of abdominal aorta is demonstrated and small HCC (arrow) is seen in right hepatic lobe. Both examiners identified lesion in image. B. Arterial-phase MR image in different transverse plane shows zebra effect of spleen, strong enhancement of abdominal aorta, and HCC (arrow) in right hepatic lobe.
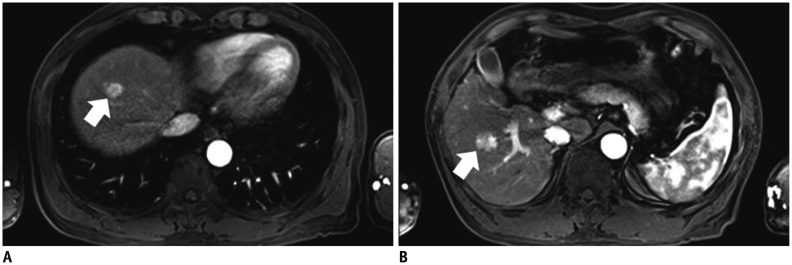
Fig. 3.
Arterial phase MR images obtained after intravenous injection of gadoxetic acid at rate of 0.5 mL/s.
A, B. 58-year-old man with hepatocellular carcinoma (HCC) and liver cirrhosis. A. Arterial-phase three-dimensional gradient-echo (repetition time/echo time = 3.4/1.3 ms; flip angle = 13°) MR images obtained after intravenous injection of gadoxetic acid at rate of 1 mL/s show strong enhancement of abdominal aorta and small HCC (arrow) in right hepatic lobe. Both examiners identified lesion in image. B. Arterial-phase MR image in different transverse plane shows zebra effect of spleen, strong enhancement of abdominal aorta, and small HCC (arrow) in right hepatic lobe.
Sensitivity for HCC Detection
Patients with another intrahepatic tumor (n = 43) such as hemangioma, metastatic tumor, or angiosarcoma were excluded from the analysis, and patients with more than five HCCs (n = 6) were also excluded from the analysis. Therefore, 47 HCCs in 36 patients with liver cirrhosis were analyzed; the mean (range) tumor diameter was 3.0 (0.7-8.5) cm. Twenty-one HCCs (in 18 patients) with a mean (range) diameter of 2.9 (0.7-8.5) cm were diagnosed in the 0.5 mL/s group.
The overall sensitivity of each method for HCC detection is described in Table 3. Examiner 1 detected 18 of the 21 HCCs in the 0.5 mL/s group and 23 of the 26 HCCs in the 1 mL/s group. Examiner 2 detected 18 of 21 HCCs in the 0.5 mL/s group and 22 of the 26 HCCs in the 1 mL/s group. No significant differences in the sensitivity for HCC detection were noted between the examiners or between the groups (p = 1.0).
Table 3.
Sensitivity for HCC Detection by Examiners
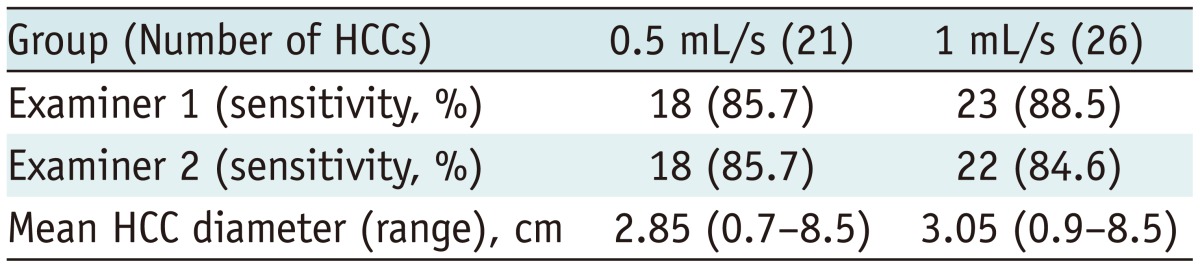
Note.-HCC = hepatocellular carcinoma
Three HCCs undetected by both readers on the arterial phase in the 0.5 mL/s group were isointense lesions. In the 1 mL/s groups, no readers detected three isointense HCCs, and one HCC undetected on the arterial phase by examiner 2 was a perceptually missed case. HCCs shown as isointense on the arterial phase were detected by low SI on the hepatobiliary phase.
Qualitative Analysis
The results of the qualitative image analysis are shown in Table 4. Subjective analyses of nine items used to assess adequate image quality (artifacts, good abdominal-aortic enhancement, strong hepatic-arterial enhancement, partial portal-venous enhancement, absence of hepatic-venous enhancement, minimal enhancement of the liver, zebra effect of the spleen, good pancreatic enhancement, and good renal corticomedullary differentiation) revealed no significant statistical difference between the two groups for the two examiners (p = 0.082-1.0).
Table 4.
Results of Qualitative Analysis of Hepatic Arterial-Phase Images Obtained Using Two Different Injection Rates of Gadoxetic Acid
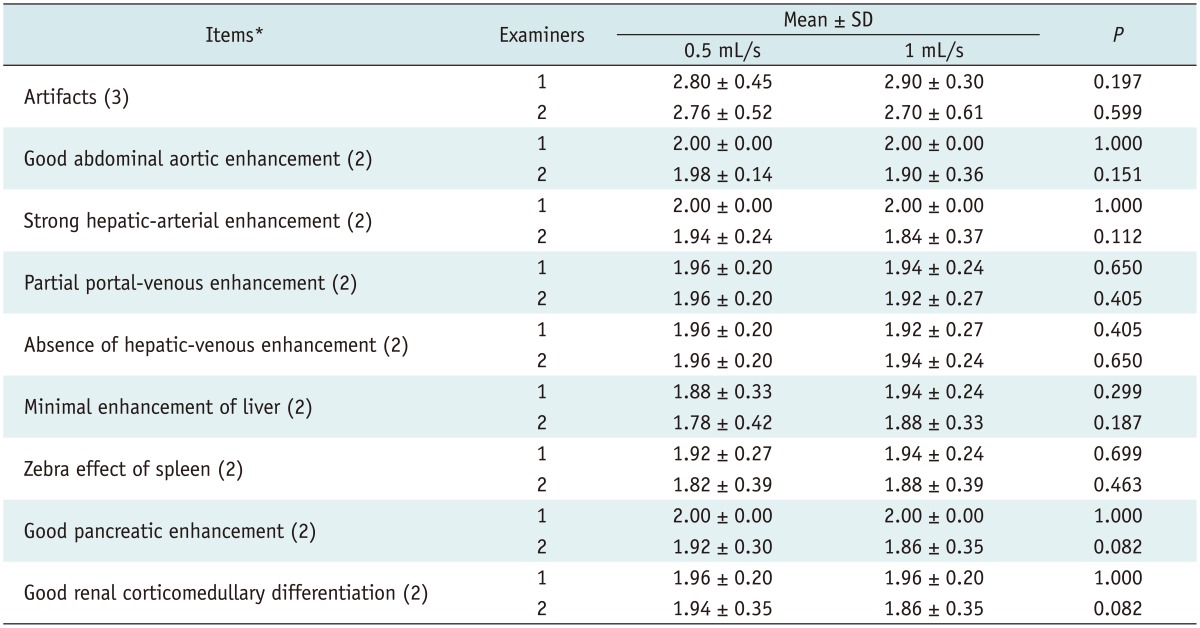
Note.-*Values in parentheses indicate maximal score of items. SD = standard deviation
DISCUSSION
This study showed that the SNRs of the liver, aorta, portal vein, spleen, and pancreas in the 0.5 mL/s group were higher than those of the 1 mL/s group, without significant differences between the two groups. However, an injection rate of 0.5 mL/s yielded slightly better enhancement of the abdominal solid organs including the liver, spleen, and pancreas, compared to the 1 mL/s injection rate during gadoxetic acid-enhanced dynamic liver MR imaging. These results may be related to the following theory. Enhancement on dynamic liver MR imaging is affected by several factors such as the patient's condition, contrast medium, and scanning technique. With regard to the contrast medium, three criteria are important for proper arterial-phase enhancement. First, the central k-space filling should match the enhancement peak. Second, gadoxetic acid shows weak protein binding in human plasma and its bound fraction has a higher relaxivity than the unbound fraction (21); therefore, sufficient protein binding of the bolus should be achieved. Finally, saturation effects in a highly concentrated bolus are responsible for a non-linear relationship between the gadolinium concentration and the MR signal; therefore, such effects should be avoided (19).
By using a slower injection rate, the bolus is stretched. This increases the probability of achieving the proper timing of an arterial phase, which results in correct filling of the central k-space in the imaging sequence, regardless of the bolus timing. A stretched bolus can also decrease artifacts resulting from varying SIs during central k-space acquisition, by improving protein binding of the contrast medium in plasma and resulting in higher relaxivity while avoiding saturation effects (21). As a result, using a slower injection rate can yield better enhancement of abdominal solid organs including the liver, spleen, and pancreas, compared to a faster injection rate during gadoxetic acid-enhanced dynamic liver MR imaging. In contrast, although the difference between the two groups was not significant, the lower tumor-to-liver CNR of HCCs in the 0.5 mL/s group is unfavorable and it can be explained by the relatively higher SI of the liver in the 0.5 mL/s group compared to that of the 1 mL/s group.
This study showed that the sensitivity for HCC detection were also not significantly different between the two injection rates. Optimal arterial phase MR imaging is important to detect hypervascular lesions such as HCC. These results suggest that the detection of HCC is possible with injection rate of 0.5 mL/s.
Additionally, the image quality scores between the examiners were not significantly different. The findings in the two groups are attributable to the optimized scan delay by using the bolus-tracking technique. However, if the same amount of the contrast agent is injected at 0.5 mL/s, the injection duration will be twice as that of a 1 mL/s injection. If a fixed scan delay is used for arterial-phase imaging, the optimal scan window for the arterial-phase scan is easier to select with an injection rate of 0.5 mL/s.
Currently, we performed gadoxetic acid-enhanced dynamic liver MR imaging with an injection rate of 0.5 mL/s combined with a bolus-tracking method, in all patients suspected of focal liver lesions from findings on ultrasonography, CT, or laboratory evaluations.
This study has two limitations. First, the presence or absence of underlying chronic liver disease, which might influence hepatic enhancement, was not considered in the quantitative or qualitative image analyses. Second, a whole series of dynamic MRI scans with injection rates of 0.5 and 1 mL/s could not be compared in the same patient.
In conclusion, image quality and sensitivity for hepatic HCCs of arterial-phase gadoxetic acid-enhanced MR would not be significantly improved by reducing the contrast injection rate to 0.5 mL/s compared with 1 mL/s.
References
- 1.Jang HJ, Yu H, Kim TK. Imaging of focal liver lesions. Semin Roentgenol. 2009;44:266–282. doi: 10.1053/j.ro.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Ba-Ssalamah A, Uffmann M, Saini S, Bastati N, Herold C, Schima W. Clinical value of MRI liver-specific contrast agents: a tailored examination for a confident non-invasive diagnosis of focal liver lesions. Eur Radiol. 2009;19:342–357. doi: 10.1007/s00330-008-1172-x. [DOI] [PubMed] [Google Scholar]
- 3.Bartolozzi C, Cioni D, Donati F, Lencioni R. Focal liver lesions: MR imaging-pathologic correlation. Eur Radiol. 2001;11:1374–1388. doi: 10.1007/s003300100845. [DOI] [PubMed] [Google Scholar]
- 4.Hamm B, Staks T, Mühler A, Bollow M, Taupitz M, Frenzel T, et al. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology. 1995;195:785–792. doi: 10.1148/radiology.195.3.7754011. [DOI] [PubMed] [Google Scholar]
- 5.Reimer P, Rummeny EJ, Shamsi K, Balzer T, Daldrup HE, Tombach B, et al. Phase II clinical evaluation of Gd-EOB-DTPA: dose, safety aspects, and pulse sequence. Radiology. 1996;199:177–183. doi: 10.1148/radiology.199.1.8633143. [DOI] [PubMed] [Google Scholar]
- 6.Vogl TJ, Kümmel S, Hammerstingl R, Schellenbeck M, Schumacher G, Balzer T, et al. Liver tumors: comparison of MR imaging with Gd-EOB-DTPA and Gd-DTPA. Radiology. 1996;200:59–67. doi: 10.1148/radiology.200.1.8657946. [DOI] [PubMed] [Google Scholar]
- 7.Reimer P, Rummeny EJ, Daldrup HE, Hesse T, Balzer T, Tombach B, et al. Enhancement characteristics of liver metastases, hepatocellular carcinomas, and hemangiomas with Gd-EOB-DTPA: preliminary results with dynamic MR imaging. Eur Radiol. 1997;7:275–280. doi: 10.1007/s003300050150. [DOI] [PubMed] [Google Scholar]
- 8.Huppertz A, Balzer T, Blakeborough A, Breuer J, Giovagnoni A, Heinz-Peer G, et al. Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology. 2004;230:266–275. doi: 10.1148/radiol.2301020269. [DOI] [PubMed] [Google Scholar]
- 9.Bluemke DA, Sahani D, Amendola M, Balzer T, Breuer J, Brown JJ, et al. Efficacy and safety of MR imaging with liver-specific contrast agent: U.S. multicenter phase III study. Radiology. 2005;237:89–98. doi: 10.1148/radiol.2371031842. [DOI] [PubMed] [Google Scholar]
- 10.Huppertz A, Haraida S, Kraus A, Zech CJ, Scheidler J, Breuer J, et al. Enhancement of focal liver lesions at gadoxetic acid-enhanced MR imaging: correlation with histopathologic findings and spiral CT--initial observations. Radiology. 2005;234:468–478. doi: 10.1148/radiol.2342040278. [DOI] [PubMed] [Google Scholar]
- 11.Halavaara J, Breuer J, Ayuso C, Balzer T, Bellin MF, Blomqvist L, et al. Liver tumor characterization: comparison between liver-specific gadoxetic acid disodium-enhanced MRI and biphasic CT--a multicenter trial. J Comput Assist Tomogr. 2006;30:345–354. doi: 10.1097/00004728-200605000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Hammerstingl R, Huppertz A, Breuer J, Balzer T, Blakeborough A, Carter R, et al. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol. 2008;18:457–467. doi: 10.1007/s00330-007-0716-9. [DOI] [PubMed] [Google Scholar]
- 13.Raman SS, Leary C, Bluemke DA, Amendola M, Sahani D, McTavish JD, et al. Improved characterization of focal liver lesions with liver-specific gadoxetic acid disodium-enhanced magnetic resonance imaging: a multicenter phase 3 clinical trial. J Comput Assist Tomogr. 2010;34:163–172. doi: 10.1097/RCT.0b013e3181c89d87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinmann HJ, Brasch RC, Press WR, Wesbey GE. Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent. AJR Am J Roentgenol. 1984;142:619–624. doi: 10.2214/ajr.142.3.619. [DOI] [PubMed] [Google Scholar]
- 15.Brasch RC, Weinmann HJ, Wesbey GE. Contrast-enhanced NMR imaging: animal studies using gadolinium-DTPA complex. AJR Am J Roentgenol. 1984;142:625–630. doi: 10.2214/ajr.142.3.625. [DOI] [PubMed] [Google Scholar]
- 16.Hendrick RE, Haacke EM. Basic physics of MR contrast agents and maximization of image contrast. J Magn Reson Imaging. 1993;3:137–148. doi: 10.1002/jmri.1880030126. [DOI] [PubMed] [Google Scholar]
- 17.Brugières P, Gaston A, Degryse HR, Parizel PM, de Schepper AM, Berry I, et al. Randomised double blind trial of the safety and efficacy of two gadolinium complexes (Gd-DTPA and Gd-DOTA) Neuroradiology. 1994;36:27–30. doi: 10.1007/BF00599189. [DOI] [PubMed] [Google Scholar]
- 18.Chung SH, Kim MJ, Choi JY, Hong HS. Comparison of two different injection rates of gadoxetic acid for arterial phase MRI of the liver. J Magn Reson Imaging. 2010;31:365–372. doi: 10.1002/jmri.22057. [DOI] [PubMed] [Google Scholar]
- 19.Schmid-Tannwald C, Herrmann K, Oto A, Panteleon A, Reiser M, Zech C. Optimization of the dynamic, Gd-EOB-DTPA-enhanced MRI of the liver: the effect of the injection rate. Acta Radiol. 2012;53:961–965. doi: 10.1258/ar.2012.120186. [DOI] [PubMed] [Google Scholar]
- 20.Zech CJ, Vos B, Nordell A, Urich M, Blomqvist L, Breuer J, et al. Vascular enhancement in early dynamic liver MR imaging in an animal model: comparison of two injection regimen and two different doses Gd-EOB-DTPA (gadoxetic acid) with standard Gd-DTPA. Invest Radiol. 2009;44:305–310. doi: 10.1097/rli.0b013e3181a24512. [DOI] [PubMed] [Google Scholar]
- 21.Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40:715–724. doi: 10.1097/01.rli.0000184756.66360.d3. [DOI] [PubMed] [Google Scholar]
- 22.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trevisani F, D'Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570–575. doi: 10.1016/s0168-8278(00)00053-2. [DOI] [PubMed] [Google Scholar]
- 24.Korean Liver Cancer Study Group and National Cancer Center, Korea. [Practice guidelines for management of hepatocellular carcinoma 2009] Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]