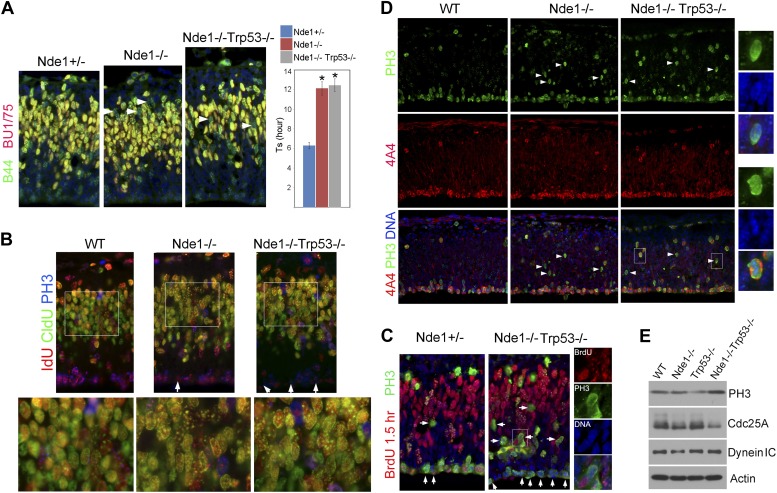
Figure 6. Persistent cell cycle stress and genotoxicity in Nde1 mutants after p53 abrogation.
(A) Representative images of B44 (green, recognizes both IdU and BrdU) and BU1/75 (red, recognizes only BrdU) double immunostained neocortical sections from IdU (2 hr), BrdU (30 min) sequential labeling experiments to measure S phase duration (Ts). Cells that were stalled in the S phase zone (IdU+ BrdU−; green) are indicated by white arrows. Significant overall difference in Ts was found among wild-type, Nde1−/−, and Nde1−/−Trp53−/− progenitors by ANOVA (p < 0.0001). Pairwise comparisons indicated that Ts of Nde1−/−Trp53−/− progenitors was significantly longer than that of wild-type progenitors (*p < 0.0001) but not significantly different from that of the Nde1−/− progenitors (n.s., p = 0.74). Data are presented as mean ± SD. (B) Representative images of B44/IdU (red), BU1/75/CldU (green), and PH3 (blue) triple immunostaining of neocortical sections from embryos sequentially labeled by IdU (2 hr) and CldU (30 min). Note the enhanced CldU immunosignals at heterochromatin structures (higher magnification views) and reduced IdU immunosignals in PH3+ cells (arrows) in Nde1−/− and Nde1−/−Trp53−/− cortical neural progenitors. (C) Immunohistological analysis of BrdU (red)–PH3 (green) co-labeled cells 1.5 hr after BrdU pulse. Arrows indicate PH3+ cells with very few BrdU foci, suggesting hindered BrdU incorporation at the end of S phase. Higher magnification views of a BrdU–PH3 double positive Nde1−/−Trp53−/− cell with uncondensed DNA are also shown. (D) Double immunostaining with G2/M marker PH3 (green) and M phase marker phospho-vimentin 4A4 (red) showing increased PH3+4A4− G2 population in Nde1−/− and Nde1−/−Trp53−/− progenitors (arrows). Higher magnification views of selected Nde1−/−Trp53−/− cells are included to show uncondensed DNA in PH3+4A4− but condensed DNA in PH3+4A4+ cells. (E) Immunoblotting analysis of neocortical lysates showing elevated PH3 and increased Cdc25A degradation in the Nde1−/−Trp53−/− mutant brain. β-actin (Actin) and dynein intermediate chain (Dynein IC) were used as loading controls.