Abstract
A 12-month-old boy with a history of bilateral retinoblastoma refractory to systemic chemotherapy, laser therapy and cryotherapy, with excellent response to previous intra-arterial melphalan infusion, presents with active tumour deposits in the right eye. Repeat intra-arterial chemotherapy was recommended. Previous bilateral melphalan infusion was uneventful using flow-guided catheterisation technique. Direct catheterisation of the right ophthalmic artery was unsuccessful despite employment of several flow-guided and over-the-wire catheters. Superselective catheterisation of the ipsilateral middle meningeal artery was unable to identify an anastomotic connection to the ophthalmic artery; however, angiography of the anterior deep temporal artery identified an alternate route for chemotherapy infusion. The anterior deep temporal artery was successfully and safely catheterised to infuse chemotherapy into the ophthalmic artery. The anterior deep temporal artery is an important potential anastomotic connection to the ophthalmic artery that can be used safely and effectively for central retinal artery chemotherapy infusion for retinoblastoma treatment.
Background
Superselective intra-arterial (IA) chemotherapy infusion for retinoblastoma in very young patients was initially developed as an eye-preservation treatment.1 IA delivery allows a high concentration of chemotherapy to be administered in a single session performed on a come-and-go basis. Catheterisation of the ophthalmic artery (OA) for chemotherapy infusion is an effective alternative to systemic chemotherapy, laser therapy, cryotherapy and external beam radiation with an acceptable risk profile.2–7 Few procedural complications, minimal side effects from chemotherapy, reduced radiation dose techniques and a growing literature of efficacy have prompted several institutions to utilise IA chemotherapy as primary treatment of ocular retinoblastoma.7–9
While IA chemotherapy infusion into the OA is often successful, direct catheterisation of the OA using standard techniques is not always possible for a variety of reasons, including anatomic variation and vasospasm. As such, knowledge of the alternate pathways to the OA may provide useful approaches for chemotherapy infusion.10 11 We present the first case of IA chemotherapy for retinoblastoma delivered by exploiting an anastomotic connection of the OA from the anterior deep temporal (ADT) artery.
Case presentation
A 4-month-old boy was initially noted to have a haze in his eyes by his mother in family photographs. Fundoscopic evaluation demonstrated bilateral tumours, with tumour burden greater in the left eye, and the patient was started on systemic chemotherapy because he was too small for IA chemotherapy. On subsequent ophthalmological examination, the tumours had not fully responded, and at age 8 months he successfully underwent bilateral IA chemotherapy using standard flow-directed techniques to catheterise the OA directly (anterograde from the internal carotid artery (ICA)) by employing a 1.5F Magic flow-directed catheter (Balt Extrusion Inc, Montmorency, France) prepared with a 0.008 inch Mirage microwire (ev3 Inc, Irvine, California, USA) with S-shaped curve (figure 1A–C and video 1). Melphalan was administered utilising a weight-based approach.6 The procedure was well tolerated and the patient was discharged after 6 h of observation. The boy had an uncomplicated postprocedure course. Fundoscopic and MRI evaluation demonstrated significant response in both eyes. However, the tumour in the right eye remained minimally active prompting repeat therapy at age 12 months.
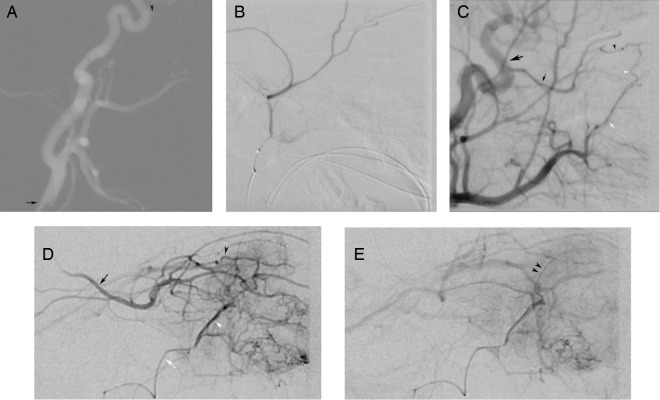
Figure 1.
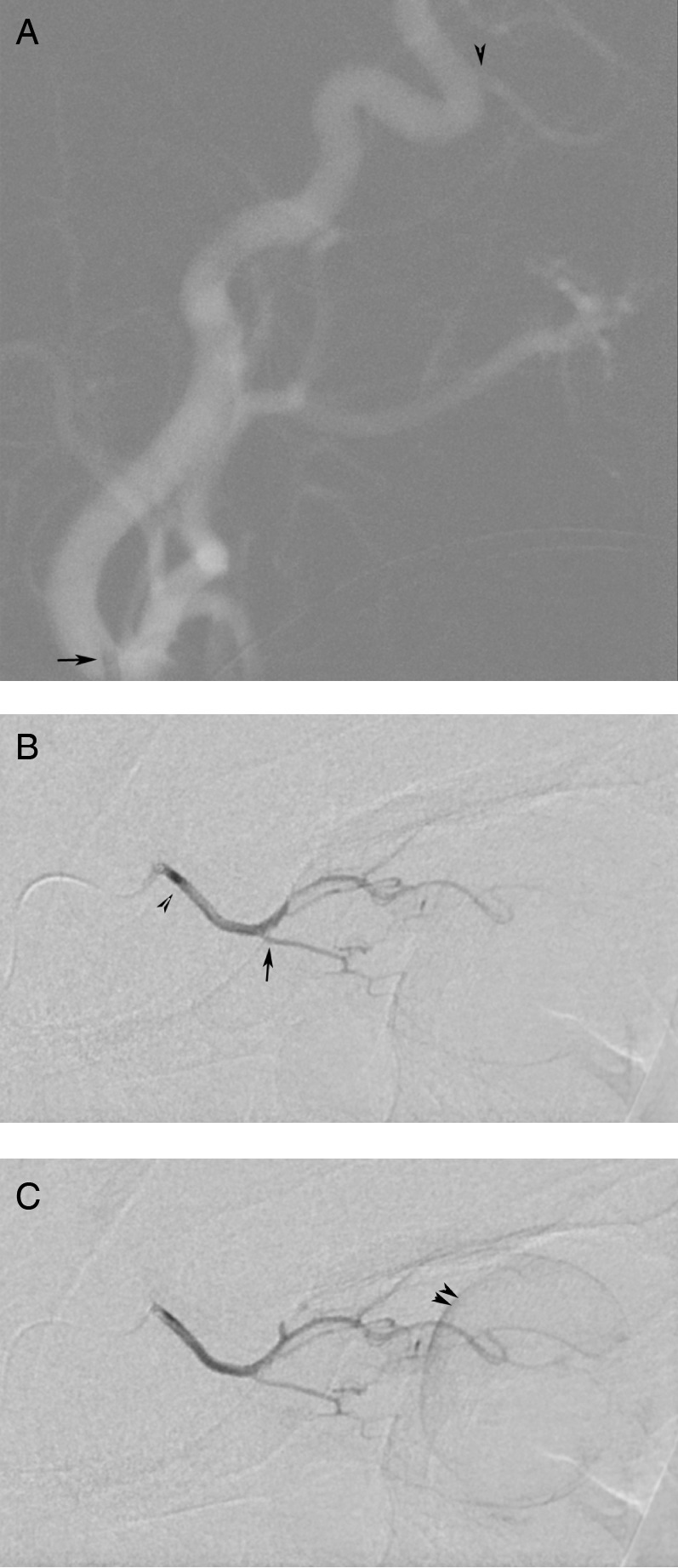
Intra-arterial administration of chemotherapy into the left ophthalmic artery (OA) was achieved using conventional methods. Lateral roadmap with the catheter tip (black arrow) positioned in the origin of the left internal carotid artery (A) identifies dorsal origin of the ophthalmic artery (black arrowhead). Early arterial phase injection of the left OA (B) demonstrates typical OA anatomy with the ciliary artery (black arrow) and the central retinal artery (not seen) arising from the ‘bayonet’ in the OA course. Late phase injection of the OA (C) demonstrates typical retinal and choroid crescent (double black arrowheads) of an adequate injection into the retinal artery.
Video of injection of the left OA. Gentle hand injection through the 1.5 F Balt Magic microcatheter positioned in the left OA origin demonstrates standard OA anatomy with central retinal artery arising from the “bayonet” curve. Later frames allow for identification of the choroidal blush as well as lacrimal branches. Frame rate is 1/sec.
Selective injection of the distal right ADT demonstrates multiple lacrimal branches that anastomose lacrimal branches of the OA. Gentle crescendo injection in the ADT allows for retrograde opacification of the ophthalmic artery (“washed-out” by unopacified blood from the right ICA in later frames), opacification of the central retinal artery, and demonstration of the choroidal blush.
Second therapeutic catheterisation of the right OA proved to be significantly more difficult. Stable position in the OA origin could not be achieved after several attempts with multiple flow-directed and over-the-wire catheters secondary to tortuosity of the carotid siphon and angulation of the proximal OA (figure 2A). An alternate route to the OA through the middle meningeal artery was considered.
Figure 2.
Intra-arterial administration of chemotherapy into the right ophthalmic artery (OA) was not successful using traditional methods of catheterisation from the internal carotid artery (ICA). Instead, the chemotherapy was administered to the artery via the anterior deep temporal (ADT) artery. Lateral roadmap with the catheter tip (black arrow) in the right common carotid artery (A) demonstrates a tortuous course of the cervical ICA as well as a dorsal OA origin (black arrowhead). Positioning in the OA was not stable. As such, the external carotid artery (ECA) was catheterised and the middle meningeal artery (MMA) was catheterised with microcatheter. Superselective injection with the catheter in the origin of the MMA (B) failed to identify the orbital branch. Selective injection of the ECA (C), with mild reflux into the ICA (large black arrow), identifies the OA (black arrow) retrograde filling from anastomotic connections between the lacrimal branches of the OA (black arrow head) and the ADT (white arrow head), which arise from the ADT (white arrow). Early arterial phase injection with the microcatheter in the distal ADT (D) demonstrates lacrimal arteries collateralising (white and black arrowheads indicating ADT and OA branches, respectively) the OA (black arrow), filling retrograde to its origin with the ICA. The ciliary and central retinal arteries are noted in the usual anatomic location at the ‘bayonet’ bend in the OA. Typical crescent (double black arrow heads) of contrast opacifying the choroid is seen in the late phase of injection (E) from the ADT signifying adequate injection into the retinal artery.
The 4F Terumo Glidecath (Terumo Corp, Tokyo, Japan) was repositioned in the external carotid artery (ECA), and the middle meningeal artery (MMA) was selectively catheterised using over-the-wire technique in an attempt to exploit a possible anastomotic connection that has been previously described.10 Unfortunately, no such connection was demonstrated on superselective angiography (figure 2B). The microcatheter was removed from the patient, and the guide catheter was positioned in the ICA to evaluate for any thromboembolic complications from the multiple attempted catheterisations.
On repeat positioning of the catheter in the ICA, mild vasospasm was encountered. Immediate subsequent interrogation of the ECA demonstrated an anastomotic connection to the OA from the ADT artery, likely exaggerated by a decrease in competitive anterograde flow in the OA (figure 2C). This prompted superselective catheterisation of the ADT using an Excelsior SL-10 microcatheter (Boston Scientific Inc, Natick, Massachusetts, USA) prepared with a Synchro2 soft preshaped microwire (Stryker Neurovascular, Fremont, California, USA).
Superselective angiography demonstrated ADT to OA anastomosis through lacrimal branches (figure 2D). Anterograde flow in the OA was overcome on the angiogram using hand injection allowing for opacification of the central retinal artery and demonstration of the choroidal blush (figure 2E and video 2). A very light hand injection was unable to overcome the force of the anterograde flow in the OA, and several test injections were performed to determine the required injection force. Weight-based dose of melphalan was subsequently administered using pulsed injection at a force greater than the established minimum force. Mid-therapy angiogram was performed to evaluate for catheter positioning and to ensure that the anterograde flow in the OA could still be overcome by the controlled hand injection as the vasospasm in the ICA was resolving. Mid-therapy angiogram demonstrated unchanged positioning of the catheter tip, and continued retrograde competitive flow in the OA allowing for opacification of the central retinal artery reassuring the operators of the technique's continued efficacy.
Post-therapy angiogram showed resolution of vasospasm in the ICA, and no evidence of thromboembolic complication. After an uncomplicated 6 h recovery period, the patient was discharged home.
Outcome and follow-up
In the perioperative period, the patient's mother noted mild periorbital bruising on the right without skin breakdown that subsequently resolved. Fundoscopic examination demonstrated the tumours had continued to decrease in size; however, very small active tumours were seen in both eyes and a third session of IA chemotherapy was recommended. There was no clinical evidence of ischaemic change to the eye or ocular adnexa.
A third session of endovascular therapy, the second bilateral treatment, was performed when the patient was 17 months of age. Traditional flow-directed methods with 1.5F Magic catheter primed with a 0.008 inch Mirage microwire allowed for stable direct catheterisation of the left OA. However, stable catheter position in the right OA was again impossible using direct catheterisation methods. The guide catheter was positioned in the ECA, and the ADT was selectively catheterised using an Excelsior SL-10 microcatheter prepared with a Synchro2 soft pre-shaped microwire. Microcatheter angiogram demonstrated the anastomotic connection to the OA, opacifying the central retinal artery and resulting choroidal blush. Catheterisation of the ADT allowed for safe and reproducible delivery of IA melphalan to the central retinal artery when OA catheterisation was unstable.
Discussion
This case is the first to illustrate feasible and reproducible chemotherapeutic delivery to the OA from the ADT artery. Prior studies have demonstrated success with IA chemotherapy through direct catheterisation of the OA; however, direct catheterisation is not always possible.10 11 Furthermore, even when catheterisation of the OA origin is achieved, this position is sometimes unstable or the flow is not entirely into the OA. In these cases, knowledge of OA anatomic and anastomotic variants is essential for safe and effective endovascular treatment of retinoblastoma.
The MMA provides the most common anastomotic and variant origin of the OA.12 13 OA supply from the MMA is from the orbital branch, which courses through the Hyrtle foramen and forms an anastomosis with the lacrimal artery near its origin with the OA.10 12–14 Similarly, the superficial temporal artery (STA) has potential anastomotic connections to the OA through the lacrimal and palpebral arteries via transverse facial and zygomatic branches, which may also be utilised for chemotherapy delivery.11
The ADT, along with the infraorbital artery, is a branch of the terminal portion of the internal maxillary artery with branches that can anastomose the OA at the level of the lacrimal gland. Anatomically, the ADT may anastomose the OA through the following three different routes: (A) the ADT may enter the orbit either through the zygomaticotemporal foramen and course superolateral to the lacrimal gland and anastomose lacrimal branches of the OA, as seen in this case, (B) the ADT may penetrate the greater wing of the sphenoid and course inferolateral within the orbit to the lacrimal gland and anastomose lacrimal branches of the OA and (C) the ADT may anastomose the STA that also supplies the supraorbital tissues where it can anastomose the supraorbital branch of the OA.15 In each of these cases, the flow through the ADT anastomotic connection has to be able to overcome the anterograde flow in the OA in order for adequate reflux of the chemotherapeutic agent in the OA to the level of the central retinal artery. Therefore, the transzygomatic and transsquamosal approaches, whose shorter course facilitates transmission of the injection pressure wave, are more likely to be functional anastomoses for the interventionalist delivering IA chemotherapy in the case of retinoblastoma.
By inadvertently causing vasospasm in the ICA, the ADT anastomosis to the lacrimal artery was accentuated allowing for successful catheterisation and chemotherapy administration. On subsequent treatment, the ADT was again catheterised after standard OA catheterisation (anterograde from the ICA) methods had failed. Even without accentuating the anastomosis by decreasing the velocity of flow in the ICA, we were able to successfully and safely administer the medication to the central retinal and ciliary arteries via this anatomic variant. Extracranial-to-intracranial anastomoses are always present. However, they are not always seen in global injections due to a haemodynamic balance among the anastamotic pathways. Superselective microcatheter injections are generally required to accentuate each of these normal anastomotic pathways.5 10 13
In order for chemotherapeutic delivery to the OA through anastomotic pathways to be effective, the injection rate and volume must be optimised. When utilising anastomotic connections to the OA distal to the central retinal artery, adequate force of hand injection is necessary to overcome anterograde flow in the OA. Low-velocity ‘dribbling’ of the medication into the vessel would likely result in deposition of the medication into the lacrimal gland and other periorbital tissues that are downstream from the OA and ADT. Similarly, a sustained high-pressure injection would likely reflux the entire length of the OA and reflux chemotherapy into the ICA. A fine balance must be obtained such that the force is strong enough to overcome the anterograde flow in the OA and short enough to deliver maximum agent into the central retinal artery. Therefore, a short, pulsed hand injection with force significant enough to overcome OA anterograde pressure and short enough to minimise melphalan loss into the ICA was utilised. Mid-therapy angiography was also helpful to ‘double check’ continued appropriate catheter position and injection rate and volume.
Like others, we have learned that direct catheterisation of the OA is not always possible, even in patients in which previous catheterisations have been successful.5 10 11 Several factors likely contribute to the variance in technical success, including anatomic variance in the origin of the OA (particularly dorsal origin from the ICA or anomalous origin from the MMA), small size of the OA in very young patients, small size of the carotid artery which limits the size of guide catheters and variance in local haemodynamic forces at the OA origin.5 One must also be cognisant that failed catheterisation of the OA may be secondary to vasospasm or damage incurred to the OA or ICA during the procedure. In our case, the entirety of the OA was visualised from the ADT injection and no irregularity of the vessel could be detected. Clinically, the patient did not have any change in his vision or neurological examination to suggest orbital or cerebral ischaemia, and follow-up fundoscopic examination of the retina did not suggest embolic or other ischaemic insult.
Specialised microcatheter shapes may assist in canalisation and achievement of stable positioning in the highly variable OA origin. However, knowledge of the potential anastamotic connections to the OA remains a requisite for practitioners of IA chemotherapeutic infusion for ocular retinoblastoma in very young patients.
Learning points.
Intra-arterial chemotherapy infusion for retinoblastoma can often be performed by catheterising the ophthalmic artery using flow-directed or over-the-wire catheters; however, traditional direct catheterisation techniques are not always successful.
- In cases of failed ophthalmic artery catheterisation, anastomotic pathways may be explored including:
- Internal maxillary artery
- Middle meningeal artery (anterior division via lacrimal artery)—most common.
- Anterior deep temporal artery (via lacrimal artery).
- Superficial temporal artery
- Transverse facial artery (via lacrimal artery).
- Zygomatic branch (via lacrimal artery).
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kaneko A, Suzuki S. Eye-preservation treatment of retinoblastoma with vitreous seeding. Jpn J Clin Oncol 2003;33:601–7 [PubMed] [Google Scholar]
- 2.Abramson DH, Gobin YP, Marr BP, et al. Intra-arterial chemotherapy for retinoblastoma. Ophthalmology 2012;119:1720–1; author reply 21 [DOI] [PubMed] [Google Scholar]
- 3.Abramson DH, Marr BP, Dunkel IJ, et al. Intra-arterial chemotherapy for retinoblastoma in eyes with vitreous and/or subretinal seeding: 2-year results. Br J Ophthalmol 2012;96:499–502 [DOI] [PubMed] [Google Scholar]
- 4.Gobin YP, Dunkel IJ, Marr BP, et al. Intra-arterial chemotherapy for the management of retinoblastoma: four-year experience. Arch Ophthalmol 2011;129:732–7 [DOI] [PubMed] [Google Scholar]
- 5.Jabbour P, Chalouhi N, Tjoumakaris S, et al. Pearls and pitfalls of intraarterial chemotherapy for retinoblastoma. J Neurosurg Pediatr 2012;10:175–81 [DOI] [PubMed] [Google Scholar]
- 6.Thampi S, Hetts SW, Cooke DL, et al. Superselective intra-arterial melphalan therapy for newly diagnosed and refractory retinoblastoma: results from a single institution. Clin Ophthalmol 2013;7:981–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooke DL, Stout CE, Kim WT, et al. Radiation dose reduction in intra-arterial chemotherapy infusion for intraocular retinoblastoma. J Neurointerv Surg 2014;0:1–5. [DOI] [PubMed] [Google Scholar]
- 8.Abramson DH, Dunkel IJ, Brodie SE, et al. Superselective ophthalmic artery chemotherapy as primary treatment for retinoblastoma (chemosurgery). Ophthalmology 2010;117:1623–9 [DOI] [PubMed] [Google Scholar]
- 9.Abramson DH, Francis JH, Dunkel IJ, et al. Ophthalmic artery chemosurgery for retinoblastoma prevents new intraocular tumors. Ophthalmology 2013;120:560–5 [DOI] [PubMed] [Google Scholar]
- 10.Klufas MA, Gobin YP, Marr B, et al. Intra-arterial chemotherapy as a treatment for intraocular retinoblastoma: alternatives to direct ophthalmic artery catheterization. AJNR Am J Neuroradiol 2012;33:1608–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooke D, Farid H, Kim W, et al. Zygomatico-orbital intra-arterial melphalan infusion for intraocular retinoblastoma. J Neurointerv Surg 2012;4:e16. [DOI] [PubMed] [Google Scholar]
- 12.Hayreh SS. Arteries of the orbit in the human being. Br J Surg 1963;50:938–53 [DOI] [PubMed] [Google Scholar]
- 13.Hayreh SS. Orbital vascular anatomy. Eye (Lond) 2006;20:1130–44 [DOI] [PubMed] [Google Scholar]
- 14.Perrini P, Cardia A, Fraser K, et al. A microsurgical study of the anatomy and course of the ophthalmic artery and its possibly dangerous anastomoses. J Neurosurg 2007;106:142–50 [DOI] [PubMed] [Google Scholar]
- 15.Quisling RG, Seeger JF. Orbital anastomoses of anterior deep temporal artery. Neuroradiology 1975;8:259–62 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video of injection of the left OA. Gentle hand injection through the 1.5 F Balt Magic microcatheter positioned in the left OA origin demonstrates standard OA anatomy with central retinal artery arising from the “bayonet” curve. Later frames allow for identification of the choroidal blush as well as lacrimal branches. Frame rate is 1/sec.
Selective injection of the distal right ADT demonstrates multiple lacrimal branches that anastomose lacrimal branches of the OA. Gentle crescendo injection in the ADT allows for retrograde opacification of the ophthalmic artery (“washed-out” by unopacified blood from the right ICA in later frames), opacification of the central retinal artery, and demonstration of the choroidal blush.