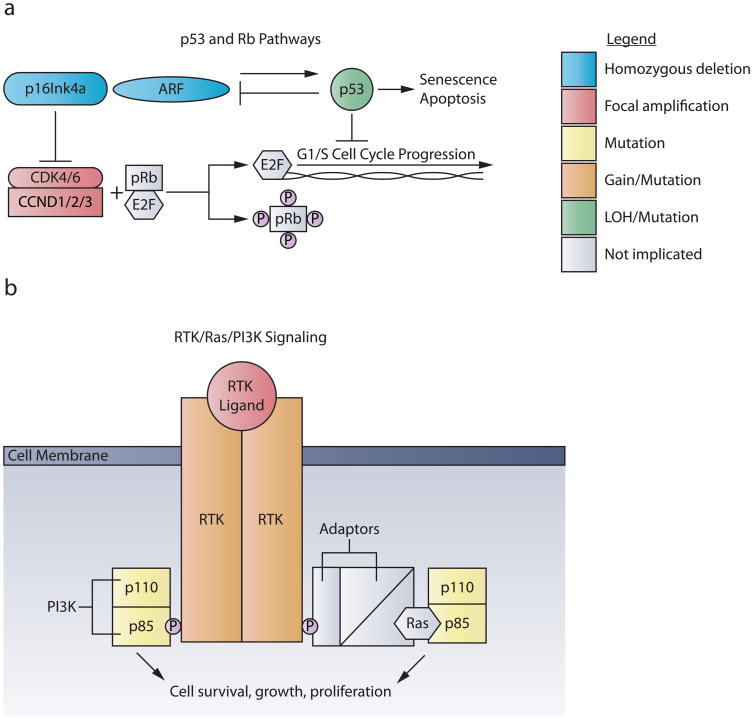
Figure 1. The p53/RB and RTK/Ras/PI3K pathways are dysregulated in pHGG.
a. The p53 and RB pathways regulate G1 cell cycle checkpoints. Mitogenic signaling activates the cyclin D-dependent kinases CKD4 or CKD6, coupled with cyclin D family members (CCND1/2/3). This complex phosphorylates pRB, releasing E2F and promoting transcription of genes responsible for G1/S cell cycle progression. Gene amplifications of CDK4, CDK6, or any of the three Cyclin D family members are found in pHGG, with greater frequency in DIPG. The tumor suppressor locus CDKN2A encodes two different proteins through translation of two different reading frames, p16INK4A and p19ARF. P16INK4A inhibits the activity of the cyclin D-dependent kinases CKD4 and CKD6. Oncogenic signals, DNA damage, or induction of P19ARF induce p53, leading to cell cycle arrest, apoptosis or senescence. Homozygous deletions of CDKN2A occur almost exclusively in NBS-HGGs; whereas TP53 mutations are common in both pNBS-HGG and DIPG.
b. Mutations in the RTK/RAS/PI3K pathway transduce unregulated signals for cell proliferation, growth and survival. RTK signaling begins when growth factor ligand binding leads to receptor dimerization. In pediatric HGG, PDGFRα is the RTK most frequently targeted by amplification and/or mutation. Upon dimerization, RTKs trans-phosphorylate one another at tyrosine residues in their cytosolic tails. p85, the regulatory subunit of PI3K, can then either directly bind to these phosphor-tyrosine residues or connect to RTKs through adaptor molecules and Ras. PI3K is comprised of catalytic (p110) and regulatory (p85) subunits, both of which are targeted by mutation, usually in a mutually exclusive pattern, in pHGG.