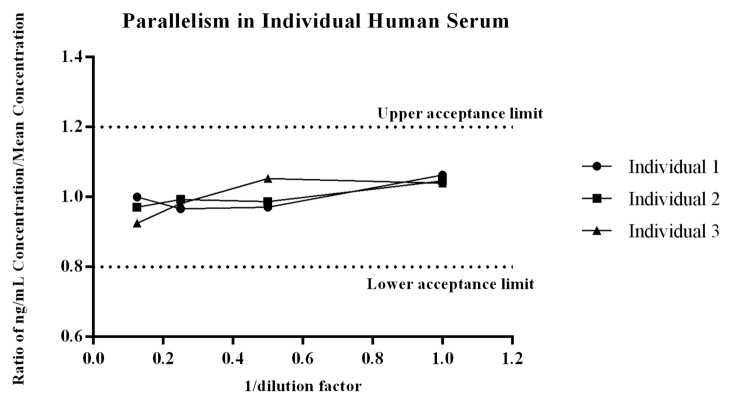
Figure 3. Parallelism of the endogenous ligand within the method was evaluated across three individual donors that were diluted at multiple dilutions and then quantitated against the recombinant PCSK9 standard curve. For each donor the concentration measured at each dilution, divided by the mean ng/mL concentration of the dilution series was assessed to determine if the ratio fell within the range of 0.8 -1.2.22 As shown all values met the acceptance criteria, giving the assay validity as a quantitative biomarker method.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.