Figure 1.
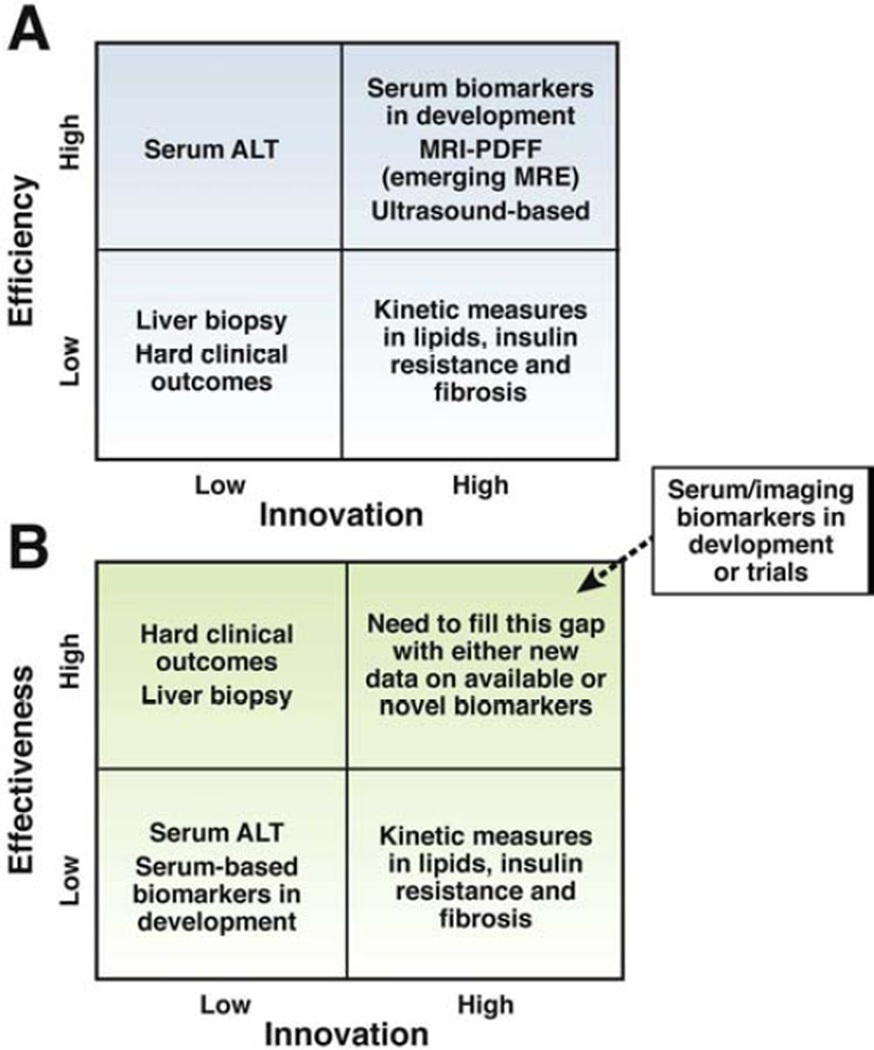
Figure A (footnote): Efficiency is needed for the conduct of phase 1 and phase 2 trials. Therefore, noninvasive biomarkers are needed. Serum ALT is an efficient biomarker for assessment of treatment response but is non-specific. Using innovative technologies, we need to strive, develop and incorporate more innovative ways of assessing treatment response in NASH to improve efficiency of early phase trials. Liver histology assessment and hard clinical outcomes such as hepatic decompensation or liver mortality are important but not practical for efficient screening for agents for drug development in early phase trials. Magnetic resonance imaging-derived proton density fat fraction, magnetic resonance elastography, and emerging ultrasound-based methods as well as emerging serum/plasma based biomarkers may be utilized for assessment of treatment response in NASH in future. High degree of innovation and high degree of efficiency needs to be matched to lead the field towards an efficient biomarker for assessing treatment response in phase 1 and 2 trials.
Figure B (footnote): Effectiveness is desirable for the conduct of phase 3 and phase 4 trials so one can credibly conclude that the therapy has unquestionable benefit in improving health in patients with NASH. Hepatic decompensation or liver mortality are clinically important end-points. However, they are not ideal because it would require a very large sample size and prolonged follow-up to show a significant benefit of treatment versus placebo due to low event rates. Therefore, innovative non-invasive biomarkers or panel of biomarkers (serum/plasma and/or imaging-based) are needed that can act as surrogate for the key hard clinical outcomes (hepatic decompensation or liver mortality) in phase 3 and 4 trials. It is advisable to incorporate these biomarkers into ongoing phase 3 trials, and future phase 4 trials in NASH.