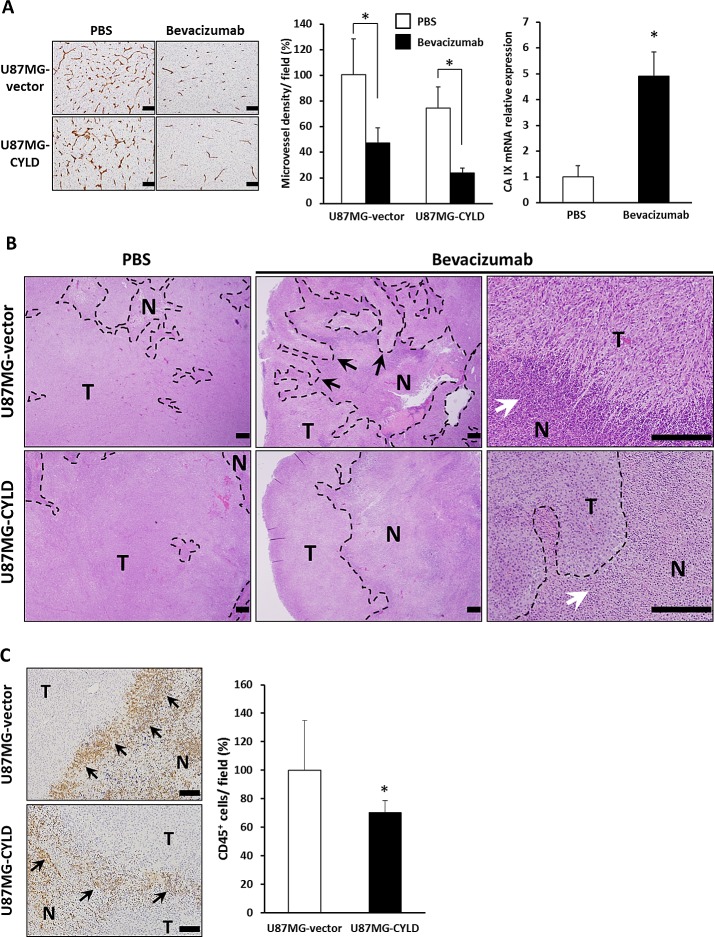
Figure 3. Effects of chronic bevacizumab treatment on histology of GBM xenografts.
(A) Sections of U87MG-vector and U87MG-CYLD tumors treated with PBS or bevacizumab were stained with an antibody against the endothelial cell surface marker CD31 (left panel). Scale bars indicate 400 μm. Microvessel density was quantified by morphometric analysis (middle panel). Values are the average of 3 to 4 independent tumors per experimental condition. CA IX mRNA expression in U87MG-vector tumors after 18 days of PBS or bevacizumab treatment was determined via qPCR (right panel). * P< 0.01. (B) Histological H&E staining analysis of tumor tissues after 18 days of PBS or bevacizumab treatment. Photomicrographs show representative examples of tumor sections in each experimental condition. N and T indicate necrotic and tumor areas, respectively. Black and white arrows indicate an invasive front and infiltration of inflammatory cells in necrotic margins, respectively. The upper right panel shows pseudopalisades. Scale bars indicate 500 μm. (C) Immunohistochemical analysis with an antibody against the inflammatory cell surface maker CD45 (left panels). CD45+ cells were quantified (right panel). Values are the average of 3 to 4 independent tumors per experimental condition. Scale bars indicate 100 μm. * P< 0.05.