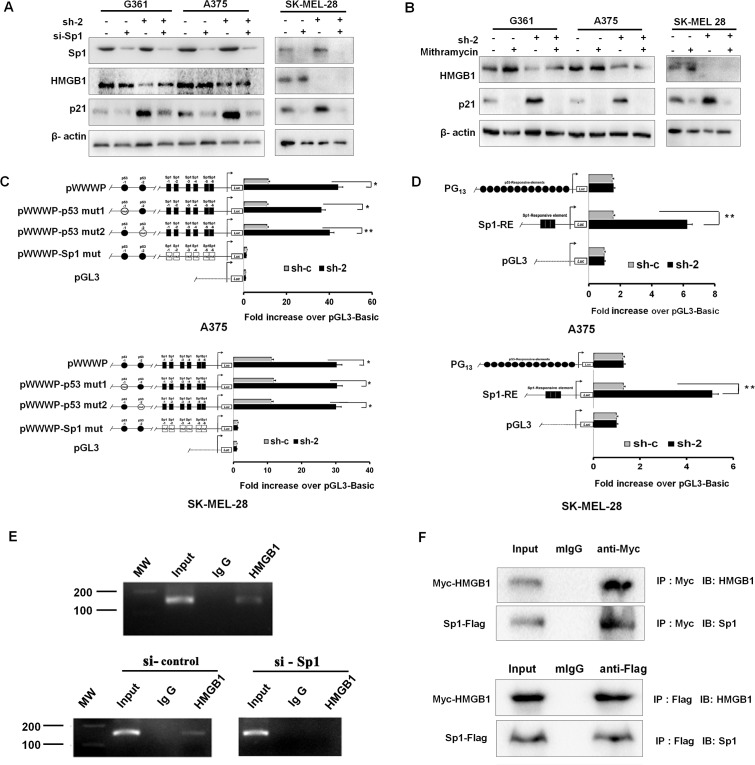
Figure 5. Upregulation of p21 induced by HMGB1 knockdown is Sp1 dependent.
(A) Control (sh-c) or HMGB1 knockdown (sh-2) A375, G361, and SK-MEL-28 cells were transfected with Sp1 siRNA (si-Sp1) or control siRNA (si-c). The cells were harvested for Western analysis of p21, HMGB1 and Sp1 expression. (B) Control or HMGB1 shRNA (sh-2) expressing A375, G361 and SK-MEL-28 cells were treated with mithramycin A (200 nM for 20 h) or control vehicle (DMSO), as indicated. The cells were harvested for Western analysis using the indicated antibodies. (C) Luciferase plasmids driving by wild-type or mutant (with p53-binding site mutated) p21 promoter were transfected into control (sh-c) or HMGB1 shRNA (sh-2) A375 (upper panel) and SK-MEL-28 (lower panel) cells. The luciferase assay was carried out 48 h post-transfection. The numbers are mean ± SD from three independent experiments. Bars, SD; *P<0.01 and **P<0.0001. (D) Similar luciferase assays but with Sp1-RE-Luc and PG13-Luc promoter plasmids were performed in A375 (upper panel) and SK-MEL-28 (lower panel). The results of three independent experiments performed in triplicate are shown. Values are mean of the ratio of Firefly luciferase activity to Renilla luciferase activity and then normalized to pGL3 vector activity. Bars, SD; *P<0.01 and **P<0.0001. (E) ChIP assays. The chromatins, which prepared from A375 cells (upper panel) and A375 cells transfected or not with si-control /si-Sp1 (lower panel), were performed with control IgG or anti-HMGB1 antibodies. The region of Sp1 binding site (spanning from −180 to −29) in the p21 promoter was amplified by PCR. (F) Plasmids expressing Myc-HMGB1 and Sp1-Flag were co-transfected into 293FT cells. Cells were harvested at 36h, and protein samples were prepared for IP with anti-Myc or anti-Flag and IgG as a control. Total cell lysates and immunoprecipitates were analyzed by Western Blots.