Abstract
Introduction:
Attention Deficit Hyperactivity Disorder (ADHD) is characterized by a persistent pattern of inattention and/or hyperactivity-impulsivity. In view of the adverse effects associated with psycho-stimulants used for the treatment of this disorder, efficacy of Brāhmī ghṛtam was evaluated in this condition.
Materials and Methods:
After following due ethical considerations, children of either sex between the age group of 6 and 12 years diagnosed to be suffering from mixed variety of ADHD as per The Diagnostic and Statistical Manual of Mental Disorders (DSM) IV criteria irrespective of other co-morbid psychiatric illnesses were recruited in the study. Initially a pilot study (n = 10) was carried out to confirm the efficacy of the identified dose of Brāhmī ghṛtam. Using this dose, further therapeutic confirmatory study (n = 27) was carried out, wherein Brāhmī ghṛtam was compared with methylphenidate. Effect on ADHD symptoms was assessed using the Dupaul ADHD rating scale and this was the main efficacy parameter.
Results:
In the pilot exploratory study, Brāhmī ghṛtam showed 66% decrease in total ADHD score. In the therapeutic confirmatory study, only 16% improvement was seen with Brāhmī ghṛtam, which was similar to methylphenidate, standard treatment for ADHD that was used as a comparator in the present study. No side-effects were reported in both studies.
Conclusion:
Our study thus has adequately demonstrated efficacy and safety of Brāhmī ghṛtam in ADHD.
KEY WORDS: Dupaul Attention Deficit Hyperactivity Disorder rating scale, psychometry
INTRODUCTION
Attention Deficit Hyperactivity Disorder (ADHD) is characterized by a persistent pattern of inattention and/or hyperactivity-impulsivity that is frequently displayed and more severe than that typically observed in individuals at a comparable level of development.[1] In the United States, ADHD is diagnosed in an estimated 8% of children between 4 and 17 years of age and in 2.9-4.4% of adults. ADHD in India is likely to have a similar prevalence to other countries.[2]
This disorder is found to be a forerunner of a number of different psychiatric conditions including mood disorders, anxiety disorders and some types of schizophrenia.[3] The symptoms of ADHD usually become evident in preschool or early years. The median age of onset of ADHD symptoms is 7 years.[4] For many individuals, ADHD symptoms improve during adolescence as age increases, but the disorder can persist into adulthood.
The modern treatment for ADHD comprises mainly of psycho-stimulant drugs such as amphetamine/methylphenidate and tricyclic antidepressants (TCAs) such as imipramine and nor-tryptylline. The major side-effects of the methylphenidate are anorexia, insomnia, loose motions etc.[5] whereas TCAs can cause anticholinergic side-effects such as dry mouth, constipation, sedation, mental confusion, increased appetite, weight gain, convulsions, and postural hypotension etc.[6] One of the most controversial issues in child psychiatry is whether the use of stimulant medications to treat ADHD increases the risk of substance abuse in adulthood. Although nonstimulants like atomoxetine HCl, which increase the levels of norepinephrine may also be used to treat ADHD in adults and children over the age of six, safety information about these drugs in long-term uses is not available.[7]
There is therefore a continuous demand for safe and effective agent to manage this condition. Brāhmī Ghṛtam is an Ayurvedic polyherbal formulation that contains four medhya (having affinity and activity on central nervous system [CNS]) plants viz. Brāhmī (Bacopa moneri), Vaca (Acorus calamus), Kuṣṭha (Sassurea lappa) and Śankhapuṣpī (Convolvulus pluricaulis) and is processed in Goghṛtam (ghee prepared from cow's milk).[8] Of these, two of the ingredients of the formulation viz. Śankhapuṣpī and Brāhmī have been earlier evaluated in different behavior disorders and have shown promising results.[9,10]
There are no direct references of this disorder in Ayurvedic texts. Some scholars correlate it with anavasthita cittatva, this term however considers only the inattention part of the disorder. Although few symptoms of Unmāda also mimic the symptoms of ADHD, the latter does not have aggravation or remission phases as can be seen in Unmāda. Since correlation of ADHD with any single disease entity described in Ayurveda is not possible, one should consider it in terms of doṣa prādhanya, which can be suggested as Vāta-pitta. While the aggravation of vāta is responsible for inattention and hyperactivity, the aggravation of pitta leads to impulsivity. The Ayurvedic management of this disorder should be planned with vāta pitta shāmaka dravyas.
The study was carried out in two phases. The first phase (pilot, exploratory) was conducted to confirm the selected dose and dosage regimen of Brāhmī ghṛtam, whereas in the second phase (therapeutic, confirmatory) Brāhmī ghṛtam was compared with methylphenidate, the standard of care in ADHD.
MATERIALS AND METHODS
Study population
Children of either sex between the age group of 6 and 12 years diagnosed to be suffering from a mixed variety of ADHD as per The Diagnostic and Statistical Manual of Mental Disorders (DSM) IV criteria irrespective of other co-morbid psychiatric illness (mild in intensity) were recruited in the study.
Mentally retarded children, those suffering from any neurological disease or from severe hepatic/cardiac/renal conditions were excluded from the study. Children who had participated in any other investigational study 30 days prior to recruitment were also excluded.
Study protocol
Pilot, exploratory study
The pilot, exploratory study was carried out at Tilak Ayurveda Mahavidyalayain collaboration with Modern High School at Pune, wherein all hyperactive children suspected to be suffering from ADHD as per the judgment of teachers of class second and third were assessed by a clinical psychologist after counseling the parents of these children. Children who satisfied the eligibility criteria were included in the study. At recruitment, detailed history of each child with focus on family history of any related disorder, socioeconomic status of the family as judged by per capita income and education of the parents was taken. Physical and systemic examination was carried out. The parents and teachers were asked to grade the performance of the child in school as poor, average and good.
The symptoms of ADHD were recorded using Dupaul ADHD rating scale. This scale also known as ADHD rating Scale-IV[11] is a reliable and user friendly instrument both for diagnosing ADHD in children and adolescents and for assessing treatment response. Containing eighteen items, the scale is linked directly to DSM-IV diagnostic criteria for ADHD. Of these items, some represent inattention, some impulsivity, whereas few items represent mixed variety.
Following this, the children were supplied with Brāhmī ghṛtam for a period of 2 months and were asked to take it in a dose of two teaspoons (~10 ml) with lukewarm water or milk around 7 a.m.–8 a.m. daily on an empty stomach. The children did not receive any other treatment even in the form of psychotherapy during this period.
During the period of 2 months, the parents were instructed to bring their children for follow-up at regular intervals of 15 days. The ADHD rating scale was re-administered at each visit. Brāhmī ghṛtam was supplied to the children for 15 days. They were provided with two days of extra supply of the medicine. They were asked to keep the bottles of Brāhmī ghṛtam tightly closed and to store in a cool, dry place. The parents were asked to record and present to us about the missed doses and overall compliance of the child.
At the end visit, all the procedures carried out at baseline were repeated. The parents were contacted 6 months after stopping the treatment to assess whether the effects of Brāhmī ghṛtam were maintained.
A child was considered as a dropout if he/she did not report for a follow-up visit for >2 days after the scheduled date of visit.
The main efficacy parameter was ADHD symptom score that was evaluated at every visit. It was further analyzed as Inattention symptom score and Impulsivity symptom score. The ancillary parameters were scholastic performance, parent feedback and palatability of the formulation, which were assessed at baseline and end visit. Number of adverse events reported at every visit was used as the safety parameter.
Therapeutic, confirmatory study
The therapeutic confirmatory study was carried out at TNMC and BYL Nair Ch. Hospital, Mumbai in an open label, randomized, comparative fashion. The study participants were recruited from psychiatry outpatient department. The eligibility criteria and baseline examinations were similar to the Pilot study. The only difference was that in addition to ADHD rating score, the children were subjected to psychometric evaluation using Mindomatics software (details available at http://www.sristek.com/products_mindomatics.asp last accessed on October 25, 2012) to test his/her cognitive functions. The tests available in the software along with their application in the area of psychometry are as follows:
Finger tapping (FT) test: This test provides information on motor system performance.
Simple reaction test (SRT): This test is useful to evaluate attention. Using this, one can assess the sensory-motor performance of the brain.
Choice reaction time (CRT): This test is useful to test attention and concentration. This test assesses sensory-motor performance of brain, and this test is considered to be a measure of psychomotor speed.
Choice discrimination test (CDT): This test is useful to test attention, integration and co-ordination. One can assess the sensory-motor performance of brain and also measure the psychomotor speed.
Digit Picture Substitution Test (DPST), Digit Letter Substitution Test (DLST), Digit Symbol Substitution Test (DSST) and Card Sorting Test (CST): These tests are useful to measure recognition of sensory information and are useful indicators of change in sensory processing performance. This tests are measures of attention, response speed, central integration, and visual motor co-ordination.
Digit vigilance task (DVTT): This test detects concentrated attention, vigilance and alertness.
Numeric working memory (NWM) and immediate picture recall test (IPRT): Both these tests are a measure of recent memory.
For each test, the software generated data about average time taken by a child for a single click, number of clicks attempted by the child and number of right as well as wrong clicks. This data was tabulated. Using these data, the error index for each test was calculated using the following formula.
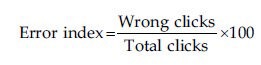
In case of FT test, the data obtained were only in form of total clicks and hence the error could not be calculated for this test.
Following the psychometric testing, the children were randomized into two groups in 2:1 proportion as per the computer generated randomization list. Children from Group 1 received Brāhmī ghṛtam in a dose of two teaspoons (~10 ml) with lukewarm water or milk to be taken in the morning, while those in Group 2 received tablet methylphenidate 0.25-1 mg/kg/day as per the judgment of the clinician, average dose being 5-10 mg/day.
The other details like schedule for follow-up visits, end visit, supply and storage of study drug, procedure for checking compliance, withdrawal and drop out criteria etc., were similar to that of the pilot study.
The efficacy and safety parameters were also similar to that of the pilot study except for the addition of psychomotor performance as an efficacy parameter. The psychomotor performance in terms of average clicking time and error index was assessed at the baseline and end of study visits.
Ethical considerations
Approvals of the Ethics Committees of both institutions were obtained prior to the study. As the study participants were from the pediatric age (7-12 years) group, written informed consent from the parents and assent from the children was taken. In addition, administrative permission from the head, Modern High School, Pune was obtained from where the study participants were screened.
Statistical analysis
Data were expressed as mean ± standard deviation in case of pilot study, the post-treatment values were compared with those of the pre-treatment. For ADHD score, Friedman's test was applied.
In case of therapeutic confirmatory study, the two treatment groups were compared with each other. For nonparametric data, Mann–Whitney test was applied. For parametric data student's unpaired t-test (continuous data) and Fisher's exact test (nominal data) were applied. The data were analyzed using Graphpad Instat version 3 (Developed by Graphpad Software, USA).
OBSERVATIONS AND RESULTS
Pilot, exploratory study
Forty-five children were screened, of whom 15 children who satisfied the eligibility criteria were recruited in the study. Of these, 10 children completed the study, while 5 children dropped out during the various stages of the study either due to time constraints on parents’ part to come for the follow-up visit or due to holidays.
None of the participants gave history of any psychiatry disorder in the family. The socioeconomic status of all participants was poor and the educational status of the parents was up to secondary school level. The mean age of the recruited children was 7.66 ± 1.32 years. The male: female ratio was 2:1.
The parents were of a similar opinion opinion about the poor performance of their child in school, lack of concentration and difficulty in writing. Five children had failed in the school academic examinations. The teachers’ opinion revealed that nine children were not performing well in academics, eight children were demonstrating behavior different than that seen in children of a similar age, such as: inability to sit at one place, follow instructions, difficulty in adjusting with siblings etc., and seven had speech related problems such as stammering.
Effect on attention deficit hyperactivity disorder score
All the children were assessed with ADHD scale at every visit. The total score obtained was subdivided into inattention and impulsivity subtypes. The results are shown in Figure 1.
Figure 1.
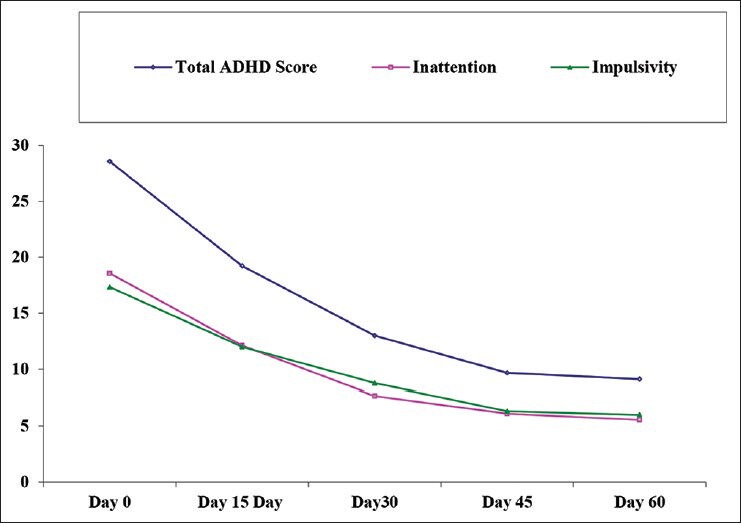
Effect of Brāhmī ghṛtam on attention deficit hyperactivity disorder score (Pilot, confirmatory study) (n = 10)
A gradual decrease was observed in total ADHD score as well as its components, which was statistically significant day 45 onwards.
Effect on ancillary parameters
The parents of all children were happy with the improvement in the behavioral findings in their child, which they had mentioned while being recruited. Most of the children liked the taste of the formulation.
No adverse event was reported.
Therapeutic confirmatory study
Forty children (20 in each study group) were recruited in the study, of which 27 children (14 from Brāhmī ghṛtam treated group and 13 from methylphenidate group) completed the study. 13 children dropped out during the various stages of the study, of which six from Brāhmī ghṛtam treated group and seven were from methylphenidate group. The same has been summarized in Figure 2.
Figure 2.
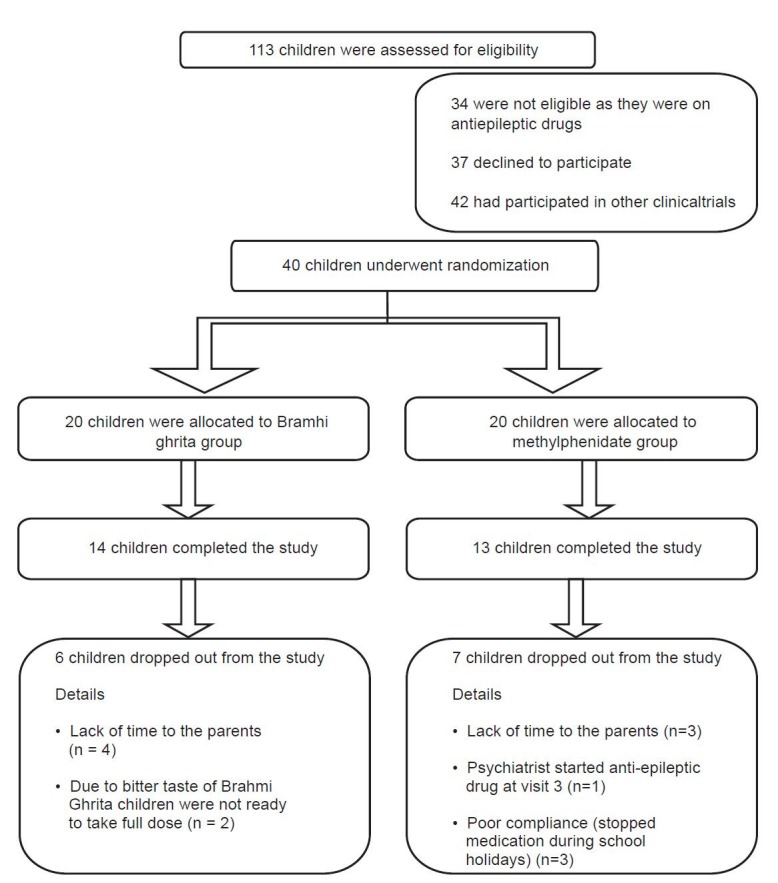
Status of study participants
The male to female ratio in Group 1 was 12:2 whereas it was 11:2 in Group 2. The mean age of children in Group 1 was 9.86 ± 1.46 years, while age of participants from Group 2 was 9.23 ± 1.59 years.
Effect on attention deficit hyperactivity disorder score
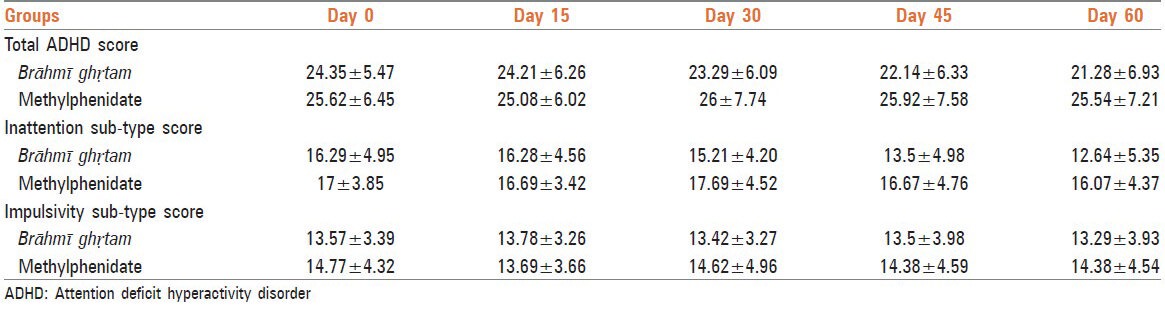
As summarized in Table 1, Brāhmī ghṛtam treated group showed gradual decrease in the total ADHD score. Although no such decrease was observed in methylphenidate group, there was no significant difference seen between the groups. Similar to total ADHD score, the inattention sub-type also showed gradual decrease in Brāhmī ghṛtam group but with no significant difference between the groups. The impulsivity sub-type however did not show such decrease.
Table 1.
Effect of Brāhmī ghṛtam on total ADHD score
Effect on psychomotor performance
The effect of the study drug on psychomotor performance was quantified as the average clicking time and percentage of error index.
It was seen that both Brāhmī ghṛtam and methylphenidate showed a reduction in the average clicking time in case of majority of tests viz. FT, choice reaction test, DPST/DLST/DSST and CST. In case of SRT and DVT, both the drugs increased the clicking time. However, in case of CDT and two tests reflecting recent memory viz. NWM and IPRT, methylphenidate showed an increase in the clicking time, while Brāhmī ghṛtam showed a reduction in the clicking time [Table 2].
Table 2.
Effect of study drug on average clicking time (ms)
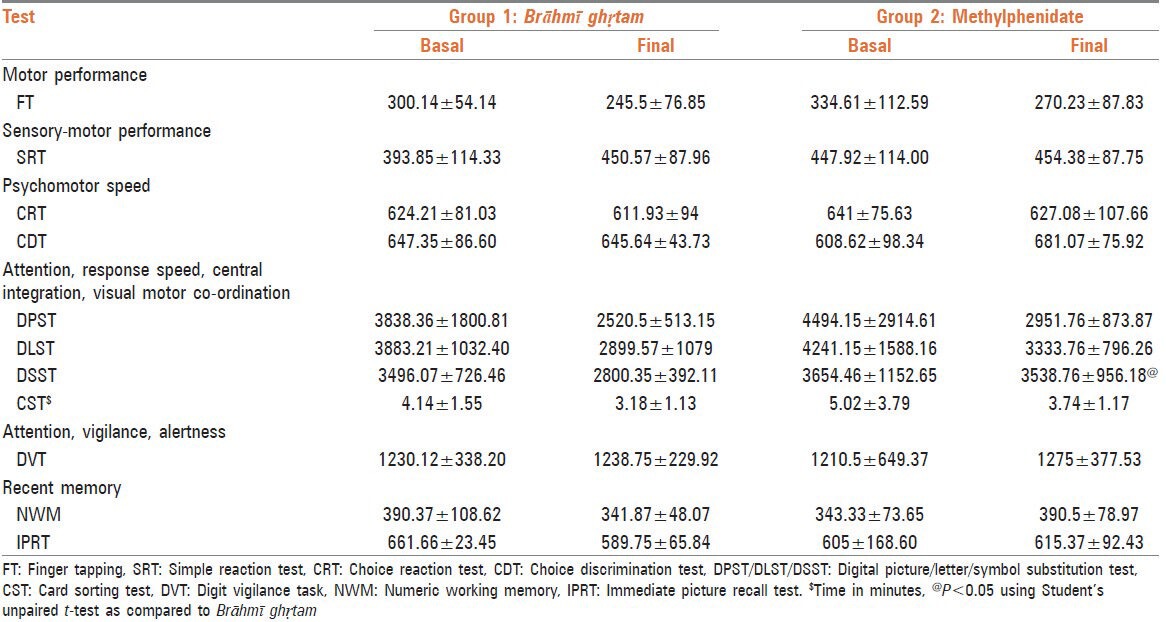
Both medications showed reduction in error index in almost all parameters, except the two tests reflecting recent memory namely NWM and IPRT where methylphenidate demonstrated increase in error index and Brāhmī ghṛtam showed reduction [Table 3].
Table 3.
Effect of error index (percentage)
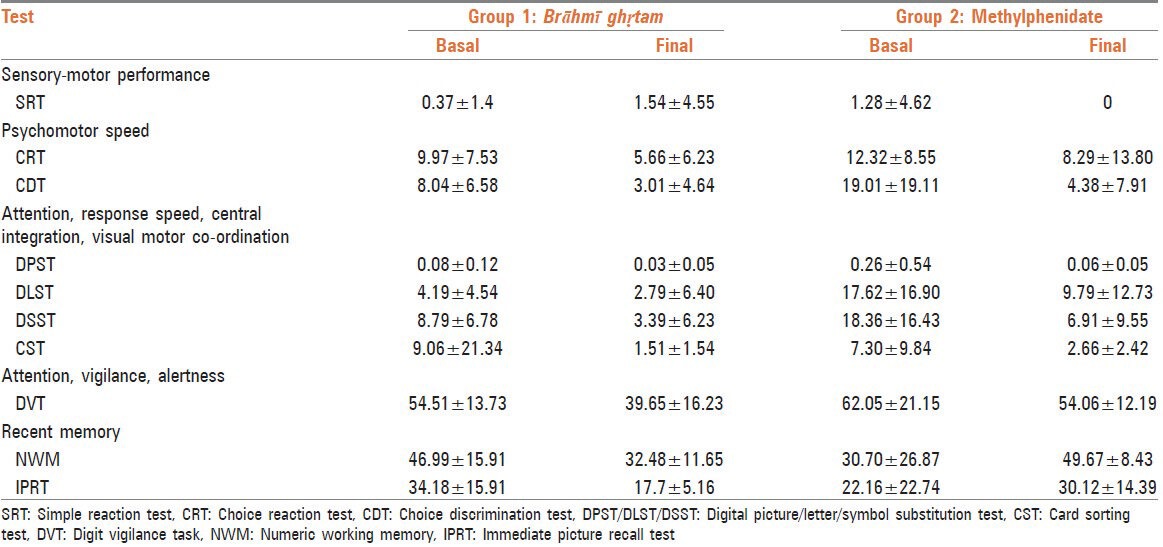
Effect on ancillary parameters
Brāhmī ghṛtam group showed significant increase in weight after treatment. The percentage change in weight in both groups was significantly different. The results are summarized in Table 4.
Table 4.
Effect on weight

Parents of nine children from Brāhmī ghṛtam treated group reported improvement in the scholastic performance (i.e. performance in the school examinations), while five parents reported no change. Parents of seven children from methylphenidate treated group reported improvement in the scholastic performance while six parents reported no change. However, there was no statistically significant difference between the groups.
Three children from Brāhmī ghṛtam group reported improvement in appetite. On the other hand, four children from the methylphenidate group reported decrease in appetite and felt the need of an appetizer along with the drug.
Out of 20 participants from Brāhmī ghṛtam group, parents of fourteen children mentioned that the medication was bitter and the children were reluctant to take it. Parents of two children asked for the formulation in capsule or tablet form.
No adverse event was reported even during this study.
DISCUSSION
We conducted two sub-studies, a pilot, exploratory study at Tilak Ayurved Mahavidyalaya (TAV), Pune and a therapeutic, confirmatory study comparing efficacy of Brāhmī ghṛtam with methylphenidate at TNMC, Mumbai. Although the objectives, study designs and the efficacy parameters were different at these sites, the primary efficacy criterion at both the places was rating of ADHD symptoms using Dupaul's ADHD rating scale. Brāhmī ghṛtam decreased the symptoms of ADHD in both the studies, though in varying degrees.
In the pilot, exploratory study at TAV, Brāhmī ghṛtam showed almost 66% decrease in total ADHD score. The ADHD score after completion of the study was significantly reduced when compared to baseline. The effect was slightly pronounced on the inattention symptoms (18.56 ± 2.69 to 5.55 ± 4.90) when compared to impulsivity symptoms (17.33 ± 4.41 to 6.00 ± 4.60) although both symptoms showed statistically significant improvement when compared to baseline.
In the therapeutic, confirmatory study at TNMC, Brāhmī ghṛtam showed 16% improvement in the ADHD score, mainly because of a decrease in the inattention symptoms. In case of both, total ADHD and inattention sub-type ADHD scores; the Brāhmī ghṛtam treated group showed significant improvement as compared to baseline. Impulsivity sub-type score was not affected by Brāhmī ghṛtam treatment. Interestingly, the response with both medications was almost similar in the various psychomotor tests carried out as a part of the study. Methylphenidate, a known CNS stimulant is reported to improve the levels of neurotransmitters in the brain, which are disturbed in ADHD leading to symptoms like hyperactivity and impulsivity.[12] Brāhmī ghṛtam may be exhibiting its effect through the same pathway. This, however, needs to be explored.
A recently published systematic review[13] on complementary and alternative medicines in ADHD has identified Bacopa monnieri (Brāhmī) as a potential agent for improving attentional and hyperkinetic disorders via a combination of cognitive enhancing and sedative effects. Although our study has demonstrated potential of Brāhmī ghṛtam in ADHD, we did not observe any sedative effect of Brāhmī; in fact the medication showed stimulant activity. This difference may be due to the presence of other plant ingredients and ghṛtam. Further studies on the mechanism of action of Brāhmī will help us understand this aspect better.
Use of Brāhmī ghṛtam has resulted in significant improvement in weight as compared to baseline, whereas the usage of methylphenidate has resulted in slight reduction in weight. Suppression of growth (i.e. weight and/or height) has been reported with the long-term use of stimulants in children though a causal relationship has not been established.[14]
On comparing the results obtained at the two sites, we can conclude that there was considerable difference in the improvement rate of ADHD symptoms. It should however be noted that methylphenidate, a standard of care for ADHD treatment also failed to show any effect on ADHD score at TNMC site. The overall poor response at the TNMC site may be due to randomized allocation of children decreasing the bias of the participants towards the intervention. Simultaneously, the possibility of a robust placebo response that is common with psychological conditions needs to be considered at TAV in view of no control. The best possible study design in this could be a three-arm study where one group receives placebo, second one receives the standard drug and the third group receives the test medicine.
In case of palatability, no child at the TAV site complained about the bitter taste of Brāhmī ghṛtam, which was a common complaint at the TNMC site. The low socioeconomic status of the participants at the TAV site depriving them from having ghrita as daily dietary substance might have influenced their response.
No side effects were reported at both the thus proving the safety of the formulation.
Attention deficit hyperactivity disorder is reported to be two to four times more common in boys than in girls.[15] A similar trend was observed in the present study. Irrespective of site and study medication, the overall male: female ratio observed in our study was 4:1.
Thus, our study has for the first time documented the possible role of Brāhmī ghṛtam in the management of ADHD. However, a small sample size and absence of placebo remain the limitations of our study. Another limitation is that it does not give any clue regarding the possible mechanism of action of Brāhmī ghṛtam. ADHD is a heterogeneous behavioral disorder with multiple possible etiologies such as neuroanatomic (brain volume reduction),[16] neurochemical (dysfunctional catecholaminergic signaling),[17] genetic basis, which further get influenced by environmental factors.[18] It will be interesting to study the effect of Brāhmī ghṛtam on these aspects.
ACKNOWLEDGMENTS
The authors sincerely acknowledge financial support received from Nair Golden Jubilee Research Foundation, TNMC. The authors also thank Dr. Ashwini Bodade and Dr. Dipti Wadangekar from TNMC and Dr. Anagha Parakhi from TAV for their help in conduct of the studies.
Footnotes
Source of Support: Nair Golden Jubilee Research Foundation, TNMC & BYL Nair Ch. Hospital, Mumbai.
Conflict of Interest: None declared.
REFERENCES
- 1.DSM-IV (Text Revision) Definition Attention-Deficit/Hyperactivity Disorder Prevalence of ADHD. [Last accessed on 2012 Oct 26]. Available from: https://www.msu.edu/course/cep/888/ADHD%20files/DSM-IV.htm .
- 2.Implementation of Climb Up®-ADHD, a Multimodal peer mediated intervention for Attention Deficit Hyperactivity Disorder in children attending a large school in Najibabad, UP. [Last accessed on 2012 Oct 26]. Available from: http://www.helpusclimbup.org/uploads/Implementation_of_Climb_Up.pdf_ClinicalTrials.gov Identifier: NCT01012778 .
- 3.Kaplan IH, Saddock JB, editors. Comprehensive Textbook of Psychiatry/V. 5th ed. II. New York, USA: Williams and Willkins Publications; 1998. Attention Deficit Hyperactivity Disorder-Adult Manifestations; p. 1782. [Google Scholar]
- 4.Median age of onset of ADHD. [Last accessed on 2012 Oct 25]. Available from: http://www.nimh.nih.gov/health/publications/the-numbers-count-mental-disorders-in-america/index.shtml#ADHD .
- 5.Hardman J, Limbard L, editors. Goodman and Gillman's The Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw Hill Medical Publication Division; 2001. Drugs acting on the central nervous system; p. 236. [Google Scholar]
- 6.Hardman J, Limbard L, editors. Goodman and Gillman's The Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw Hill Medical Publication Division; 2001. Drugs acting on the central nervous system; p. 466. [Google Scholar]
- 7.Ledbetter M. Atomoxetine: A novel treatment for child and adult ADHD. Neuropsychiatr Dis Treat. 2006;2:455–66. doi: 10.2147/nedt.2006.2.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambikadattashastri, editor. 14th ed. Published by Chaukhabha Sanskrit Sansthana; 2001. Baishjyaratnavali. Apasmar chikitsa; p. 372. [Google Scholar]
- 9.Kapse A. Study of medhya karma of Shankhpushpi with special reference to Behaviour Disorders in Adolescent age group, MD thesis submitted to Pune University. 2005 [Google Scholar]
- 10.Desai A. Study of medhya karma of Bramhi (Bacopa mooneiri) in Oppositional Defiant Disorder, MD thesis submitted to MUHS, Nasik. 2009 [Google Scholar]
- 11.DuPaul GP. Parent and Teacher Rating of ADHD Symptoms: Psychometric Properties in a Community Based Sample. J Clin Child Psychol. 1991;20:245–53. [Google Scholar]
- 12.Tomasi D, Volkow ND, Wang GJ, Wang R, Telang F, Caparelli EC, Wong C, Jayne M, Fowler JS. Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. Neuroimage. 2011;54:3101–10. doi: 10.1016/j.neuroimage.2010.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarris J, Kean J, Schweitzer I, Lake J. Complementary medicines (herbal and nutritional products) in the treatment of Attention Deficit Hyperactivity Disorder (ADHD): A systematic review of the evidence. Complement Ther Med. 2011;19:216–27. doi: 10.1016/j.ctim.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Negrao BL, Viljoen M. Stimulants and growth in children with attention-deficit/hyperactivity disorder. Med Hypotheses. 2011;77:21–8. doi: 10.1016/j.mehy.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Alobaidi AK, Ali NS. Attention Deficit/Hyperactivity Disorder among schoolchildren in Baghdad. J Can Acad Child Adolesc Psychiatry. 2009;18:4–5. [PMC free article] [PubMed] [Google Scholar]
- 16.Krain AL, Castellanos FX. Brain development and ADHD. Clin Psychol Rev. 2006;26:433–44. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Aguiar A, Eubig PA, Schantz SL. Attention deficit/hyperactivity disorder: A focused overview for children's environmental health researchers. Environ Health Perspect. 2010;118:1646–53. doi: 10.1289/ehp.1002326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gene Predicts Better Outcome as Cortex Normalizes in Teens with ADHD NIMH Press Release. 2007 Aug 6; [Google Scholar]


