Figure 2.
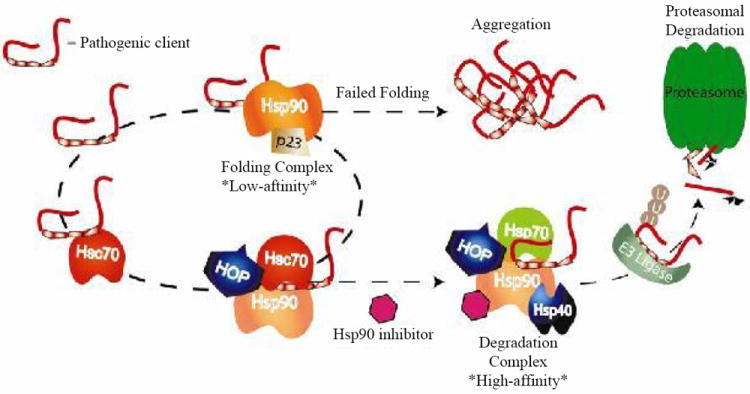
Protein folding pathways can prove to be detrimental when the chaperone folding machinery fails to properly process an aberrant aggregation-prone client protein. Under the condition of Hsp90 inhibition, Hsp90 is removed from the unproductive folding equation, disrupting this pro-aggregation pathway. Also, inhibition of Hsp90 induces the HSPs, including Hsp70. Increased levels of Hsp70 can facilitate a higher incident of interaction between client and pro-degradation pathway. As an example, interaction of a client with an ubiquitin ligase promotes proteasomal degradation of aberrant clients rather than accumulation and aggregation.
