Table 4.
Inhibition of human sEH by urea derivatives substituted with oxyoxalamide function
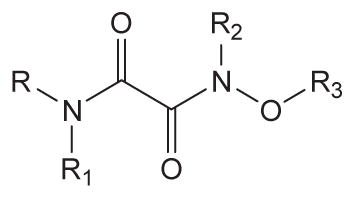
| ||||||
|---|---|---|---|---|---|---|
| No. | R | R1 | R2 | R3 | Human sEH IC50 a (nM) | Solubilityb (μM) |
| 27c |
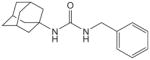
|
16 | 20 | |||
| 28 |
|
H | H | CH3 | 6.6 | 312 |
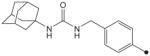
| 29 |
|
H | H |
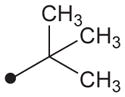
|
19 | 39 |
| 30 |
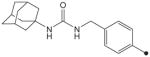
|
H | CH3 | CH3 | 5.0 | 312 |
| 31 |
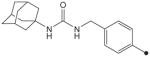
|
CH3 | CH3 | CH3 | 1.2 | 625 |
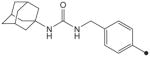
| 32 |
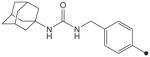
|
H | H |
|
19 | 78 |
| AUDAd | 3.2 | 63 | ||||
| IK950d | 14 | 625 | ||||
Test compounds prepared in DMSO was reacted with human sEH (1 nM) for 10 min in 25 mM Bis–Tris/HCl buffer (202 μL; pH 7.0) at 30 °C. The fluorescent substrate (CMNPC; [S] = 5 μM) was then introduced to the incubation mixture. Inhibition potency against the human sEH was determined by measuring the appearance of the 6-methoxy-2-naphthaldehyde with an excitation wavelength of 330 nm and an emission wavelength of 465 nm for 10 min on a fluorometer. Results are averages of three separate measurements. See the Supplementary data for the detailed procedures.
Water solubility was determined by adding a variety of concentrations of a test compound prepared in DMSO to 0.1 M sodium phosphate buffer (pH 7.4) in a final ratio of 5:95 (v/v). The turbidity of the water solution was measured at 650 nm to determine solubility in water. Results are the average of triplicate determinations.
Urea inhibitor with no substitution by oxyoxalamide function, which was synthesized by the reaction of 1-adamantyl isocyanate with benzyl amine in DMF in 100% yield.13
