Figure 1.
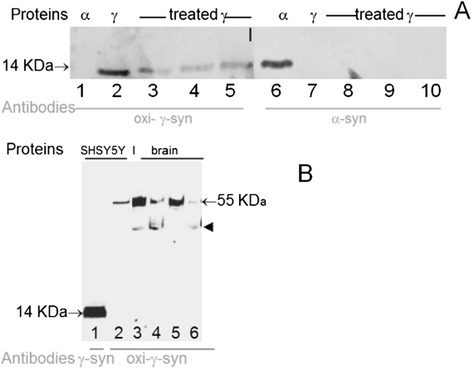
Specificity of synuclein antibody toward recombinant proteins (A) and extracts from cell culture and brain (B). A: Recombinant α-syn (lanes 1 and 6), γ-syn (lanes 2 and 7) and γ-syn treated with DA (oxi-γ-syn, lanes 3–5 and 8–10) were separated in 12% polyacrylamide gel with SDSNa and tested with antibodies specific to different synucleins isoforms. The following amounts of protein were subjected to electrophoresis: lanes 2 and 7 – untreated -γ-syn - 62.5 ng. Since in the course of the treatment by 250 μM DA the amount of immunoreactive monomeric γ-syn was reduced, we applied increasing amounts of proteins: 100 ng on lanes 3 and 8; 187.5 ng on lanes 4 and 9; and 225 ng on lanes 5 and 10. WB was probed with antibodies against oxi-γ- syn (lanes 1–5) and antisheep-α-synuclein (lanes 6–10). B: Antibody to unmodified γ-syn (ABcam, 55424) recognizes monomeric form of γ-syn (lane 1), while antibody to oxi-γ-syn recognizes tetrameric γ-syn in cell extracts of SH-SY5Y cells overexpressing γ-syn (lane 2) and in extracts of human brain (3–6). Lanes 3 and 5 extracts from amygdala were analyzed; lanes 4 and 6 – extracts from substantia nigra from control individuals.
