Figure 1.
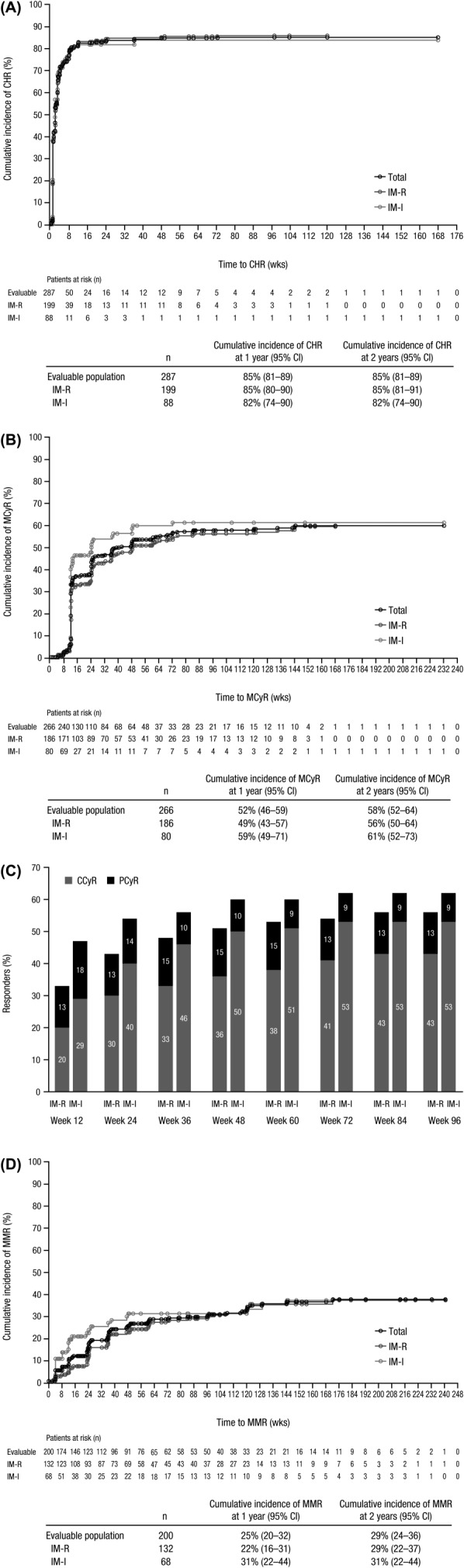
Cumulative incidence curve for time to response adjusting for the competing risk of treatment discontinuation without response. Time to CHR (A), MCyR (B), and MMR (D) was calculated among evaluable patients with a valid baseline assessment from the start date of therapy until the first date of attained/maintained response (confirmed for CHR and unconfirmed for MCyR and MMR) or last nonmissing assessment date for those without a response or discontinuation. All treated patients were evaluable for MMR except patients from sites in China, India, Russia, and South Africa, who were not assessed for molecular response. (C) Rates of MCyR, including PCyR and CCyR, were cumulative by the defined time points for evaluable patients (IM-R, n = 186; IM-I, n = 80) who had an adequate baseline cytogenetic assessment and maintained/achieved their response. Abbreviations: CCyR, complete cytogenetic response; CHR, complete hematologic response; IM-I, imatinib intolerant; IM-R, imatinib resistant; MCyR, major cytogenetic response; MMR, major molecular response; PCyR, partial cytogenetic response.
