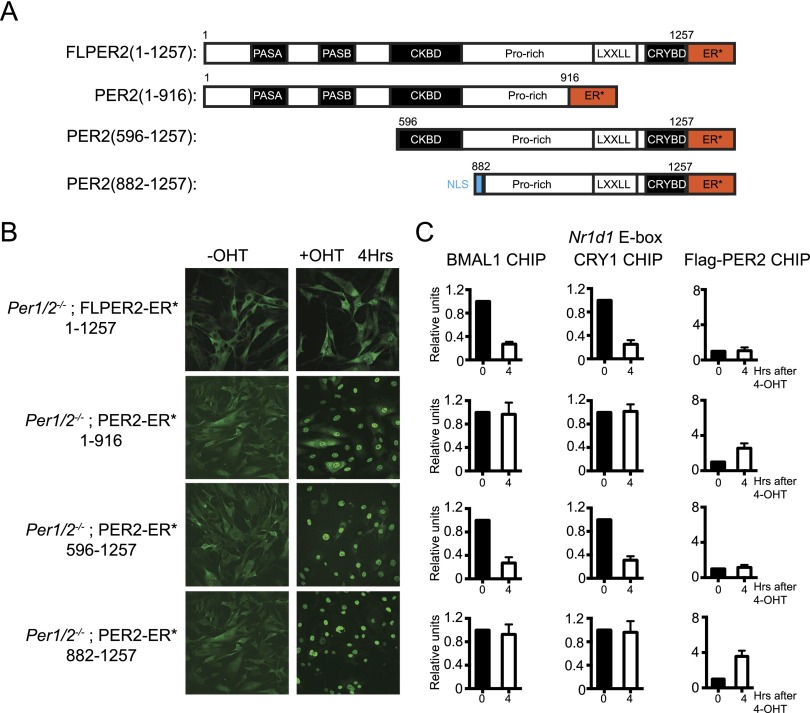
Figure 4.
Mapping of PER2 domains necessary for disrupting the CRY–CLOCK–BMAL1–promoter complex. (A) PER2-ER* constructs used in Per1/2−/− cells. The numbers associated with each mutant represent the amino acids of full-length PER (1257 amino acids) contained in each mutant protein. An SV40 nuclear localization signal is fused to the PER2(882–1257) to assure its nuclear translocation. (B) Nuclear entry of the fusion proteins following 4 h of treatment with 4-OHT analyzed by immunofluorescence. (C) Analyses of BMAL1, CRY1, and PER2 chromatin binding by ChIP. Full-length PER2 and PER2(596–1257) disrupt CRY1–CLOCK–BMAL1 binding to chromatin without measurable PER2 binding. Neither the N-terminal half [PER2(1–916)] nor the C-terminal half [PER2(882–1257)] of the protein have an effect on CRY1–CLOCK–BMAL1 binding to chromatin, but both do weakly associate with the promoter.